スルファフェナゾール 化学特性,用途語,生産方法
解説
4-amino-N-(1-phenyl-1H-pyrazol-5-yl)benzenesulfonamide.C15H14N4O2S(314.36).3-アミノ-2-フェニルピラゾリンをアセチルスルファニリルクロリドと反応させてつくる.
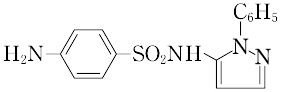
"微黄色の結晶性粉末.融点180~184 ℃.無臭でわずかに苦味をもつ.アセトンに易溶,メタノール,希塩酸,アルカリに可溶,エタノールに微溶,水に不溶.血中有効濃度の接続時間が長いので持続性サルファ剤とよばれる.髄膜炎,腎盂(じんう)炎に用いられる.LD50 4507 mg/kg(マウス,経口).[CAS 526-08-9]
森北出版「化学辞典(第2版)
効能
抗菌薬, 葉酸合成阻害薬
説明
CYP2C9 is a major cytochrome P450 enzyme that is involved in the metabolic clearance of various therapeutic agents. Disruption of this enzyme’s activity can lead to adverse drug reactions. Sulfaphenazole is an inhibitor of CYP2C9 (K
i = 0.3 μM) that demonstrates at least 100-
fold selectivity over other CYP450 isoforms (K
is = 63 and 29 μM for CYP2C8 and CYP2C18, respectively, and no activity at CYP1A1, CYP1A2, CYP3A4, CYP2C19). At 10 μM, sulfaphenazole has been shown to inhibit endothelium-
derived hyperpolarizing factor synthase, a CYP450 isozyme in the porcine coronary artery homologous to CYP2C8/9 that generates reactive oxygen species in coronary endothelial cells and modulates vascular tone and homeostasis.
化学的特性
White to off-white powder
定義
ChEBI: A sulfonamide that is sulfanilamide in which the sulfonamide nitrogen is substituted by a 1-phenyl-1H-pyrazol-5-yl group. It is a selective inhibitor of cytochrome P450 (CYP) 2C9 isozyme, and antibacterial agent.
純化方法
Crystallise it from EtOH or aqueous EtOH. [Schmidt & Druey Helv Chim Acta 41 309 1958, Beilstein 25 III/IV 2029.]
スルファフェナゾール 上流と下流の製品情報
原材料
準備製品