ファルネソール (異性体混合物) 化学特性,用途語,生産方法
外観
無色~うすい黄色, 澄明の液体
定義
本品は、次の化学式で表されるトリメチルドデカトリエノールである。
溶解性
水にほとんど溶けない。
解説
3,7,11-trimethyl-2,6,10-dodecatriene-1-ol.C15H26O(222.37).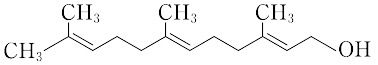 シトロネラ油,レモングラス油,シクラメン油,ばら油などの香気成分.多くの精油には,このtrans-trans形異性体のみが存在する.無色透明な液体.沸点111 ℃(47 Pa).d154"0.895.n25D"1.487.λmax 224 nm(ε 17.300).非環式セスキテルペンアルコールの一例で,種々の植物精油中に含まれる。水に不溶,アルコール,エーテルに可溶の液体で,トランス-トランス異性体の沸点は 0.2mmHgの圧力下で 120℃である。香料原料として用いられる。また,ピロリン酸エステルはカロテノイド,ステロイドの生合成経路における中間体と考えられている。森北出版「化学辞典(第2版)
シトロネラ油,レモングラス油,シクラメン油,ばら油などの香気成分.多くの精油には,このtrans-trans形異性体のみが存在する.無色透明な液体.沸点111 ℃(47 Pa).d154"0.895.n25D"1.487.λmax 224 nm(ε 17.300).非環式セスキテルペンアルコールの一例で,種々の植物精油中に含まれる。水に不溶,アルコール,エーテルに可溶の液体で,トランス-トランス異性体の沸点は 0.2mmHgの圧力下で 120℃である。香料原料として用いられる。また,ピロリン酸エステルはカロテノイド,ステロイドの生合成経路における中間体と考えられている。森北出版「化学辞典(第2版)
用途
有機合成用試薬。香料原料
化粧品の成分用途
香料、消臭剤
説明
Farnesol has a characteristic flowery odor.
化学的特性
Farnesol is a component of
many blossom oils. It is a colorless liquid with a linden blossom odor, which
becomes more intense when evaporated.
Of the four possible isomers (due to the double bonds in the 2- and 6-positions),
the (2E,6E)-isomer is the most common in nature and occurs, forexample, in ambrette seed oil.(2Z,6E)-Farnesol [3790-71-4] has been
identified in petitgrain oil bigarade.
Synthetic farnesol is a mixture of isomers obtained by isomerization of nerolidol.
Farnesol is particularly suited for use in flower compositions and is valued for
its fixative and deodorizing properties.
天然物の起源
The presence of this terpene alcohol in nature has been reported in more than 30 essential oils; the levels are generally low (0.5 to 1.0%) with the exception of cabreuva, which contains up to 2 5% farensol, and the distillate from fowers of Oxystigma buccholtzii Harms which contains up to 18% farnesol Among the essential oils containing farnesol are lemongrass, Ceylon citronella, cananga, ambrette seeds, ylang-ylang, Acacia farnesiana, Peru balsam, palmarosa, tuberose, and others Reported found in apricot, citrus peel oils, grapefruit juice, strawberry jam, ginger, clove bud, hop oil, cardamom, ginger, thyme, beer, whiskey, basil, papaya, anise seed, German chamomile and Cympogon citratus oils
使用
farnesol is described as a substance of high biological potential, capable of acting in the skin as a true bioactivator. A biological precursor and fatty alcohol, farnesol is one component of vitamin K. It is said to help smooth wrinkles, normalize sebum secretion, and increase the skin’s elasticity, tissue tension, and moisture-binding capability. It is able to penetrate the epidermis. In humans, farnesol is found in the skin and is involved in sterol biosynthesis. It is also used for its deodorant, odor-masking, and skin-soothing properties. In clinical studies, farnesol has demonstrated anti-microbial activity, though it is unclear if this remains the case once incorporated into a cosmetic formulation. It is widely present in vegetables and found in many essential oils (for example, acacia, lilac, lily of the valley, rose, orange blossom, oak moss, and sandalwood).
製造方法
One method uses cabreuva as the starting material (Swiss Patent 261,120-Givaudan and Co ), while a second method starts from ambrette seeds (German Patent 149, 603-Haarmann and Reimer).
定義
ChEBI: A farnesane sesquiterpenoid that is dodeca-2,6,10-triene substituted by methyl groups at positions 3, 7 and 11 and a hydroxy group at position 1.
一般的な説明
Colorless liquid with a delicate floral odor.
反応プロフィール
Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.
接触アレルゲン
Farnesol is one of the most frequent contact allergens
in perfumes. It is contained in small amounts in
Myroxylon pereirae and poplar buds. It is a blend of
four diastereosiomers trans/cis. As a fragrance allergen,
farnesol has to be mentioned by name in cosmetics
within the EU.
抗がん研究
A pharmacogenomic approach was used for the farnesol. Tests on many genesinvolved in apoptosis, regulation of transcription, and genes like INE1, CTRL,MRS2, NEB, LMO7, C9orf3, and EHBP1 are not conferred resistance to farnesol.The effects of farnesol on genes not related to the resistance to anticancer drugs mayspeculate the design of new drugs against tumor-resistant line (Manjamalai andGrace 2012a, 2012b; Ji et al. 2014).
純化方法
The main impurity is the cis-trans isomer. Purify it by gas chromatography using a 4ft x 0.125in 3%OV-1 column at 150o. [Corey et al. J Am Chem Soc 92 6637 1970, Popjak et al. J Biol Chem 237 56 1962.] It has also been fractionated through a 14-in Podbielniak column (p 11) at 11o/0.35mm. Alternatively it has been purified by gas chromatography using SF96 silicone on Fluoropak columns or Carbowax 20M on Fluoropak or base-washed 30:60 firebrick (to avoid decomposition, prepared by treating the firebrick with 5N NaOH in MeOH and washed with MeOH to pH 8) at 210o with Helium carrier gas at 60 mL/min flow rate. The diphenylcarbamoyl derivative has m 61-63o (from MeOH) and has an IR band at 3500 cm-1. [Bates et al. J Org Chem 28 1086 1963, Beilstein 1 IV 2335.]
ファルネソール (異性体混合物) 上流と下流の製品情報
原材料
準備製品