|
|
| | methyl (4-nitro-1H-pyrazol-1-yl)acetate Basic information |
| Product Name: | methyl (4-nitro-1H-pyrazol-1-yl)acetate | | Synonyms: | methyl (4-nitro-1H-pyrazol-1-yl)acetate;Methyl 4-Nitro-1H-pyrazole-1-acetate;methyl 2-(4-nitropyrazol-1-yl)acetate;1H-Pyrazole-1-acetic acid, 4-nitro-, methyl ester | | CAS: | 6715-84-0 | | MF: | C6H7N3O4 | | MW: | 185.14 | | EINECS: | | | Product Categories: | | | Mol File: | 6715-84-0.mol | 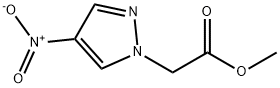 |
| | methyl (4-nitro-1H-pyrazol-1-yl)acetate Chemical Properties |
| Boiling point | 321.7±22.0 °C(Predicted) | | density | 1.48±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | pka | -2.71±0.10(Predicted) | | Appearance | White to off-white Solid | | InChI | InChI=1S/C6H7N3O4/c1-13-6(10)4-8-3-5(2-7-8)9(11)12/h2-3H,4H2,1H3 | | InChIKey | GEADOFDFVOSIOA-UHFFFAOYSA-N | | SMILES | N1(CC(OC)=O)C=C([N+]([O-])=O)C=N1 |
| | methyl (4-nitro-1H-pyrazol-1-yl)acetate Usage And Synthesis |
| Uses | Methyl (4-Nitro-1H-pyrazol-1-yl)acetate is used in preparation of novel substituted glycine derivatives as Factor XIa and/or plasma kallikrein inhibitors. | | Synthesis | Step 1: To a solution of 4-nitro-1H-pyrazole (100 g, 0.88 mol) in acetonitrile (ACN, 2 L) was added potassium carbonate (K2CO3, 183.2 g, 1.33 mol) and methyl chloroacetate (95.6 g, 0.88 mol). The reaction mixture was heated to 60 °C and the reaction was stirred at this temperature for 5 hours. Upon completion of the reaction, the mixture was filtered to remove insoluble solids and the solvent was subsequently removed by distillation under reduced pressure to afford methyl 2-(4-nitro-1H-pyrazol-1-yl)acetate as a white solid (150 g, 92% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 8.28 (s, 1H), 8.11 (s, 1H), 4.98 (s, 2H), 3.84 (s, 3H). | | References | [1] Patent: WO2013/14162, 2013, A1. Location in patent: Page/Page column 99 |
| | methyl (4-nitro-1H-pyrazol-1-yl)acetate Preparation Products And Raw materials |
|