- BocNH-PEG3-CH2COOH
-

- $23.28 / 1g
-
2026-01-24
- CAS:462100-06-7
- Min. Order: 1g
- Purity: 98%
- Supply Ability: 300kg
- Boc-NH-PEG3-CH2COOH
-

- $29.00 / 100mg
-
2026-01-23
- CAS:462100-06-7
- Min. Order:
- Purity: ≥98%
- Supply Ability: 10g
- Boc-NH-PEG3-CH2COOH
-

- $29.00 / 100mg
-
2026-01-23
- CAS:462100-06-7
- Min. Order:
- Purity: ≥98%
- Supply Ability: 10g
|
| | 2,2-DIMETHYL-4-OXO-3,8,11,14-TETRAOXA-5-AZAHEXADECAN-16-OIC ACID Basic information |
| Product Name: | 2,2-DIMETHYL-4-OXO-3,8,11,14-TETRAOXA-5-AZAHEXADECAN-16-OIC ACID | | Synonyms: | 5,8,11-Trioxa-2-azatridecanedioic acid 1-(1,1-dimethylethyl) ester;Boc-N-amido-PEG3-CH2CO2H;Boc-PEG3- CH2COOH;Boc-11-amino-3,6,9-trioxaundecanoic acid;t-boc-N-amido-PEG3-acetic acid;BocNH-PEG3-CH2COOH;2-[2-[2-[2-(Boc-amino)ethoxy]ethoxy]ethoxy]acetic Acid;2,2-DIMETHYL-4-OXO-3,8,11,14-TETRAOXA-5-AZAHEXADECAN-16-OIC ACID | | CAS: | 462100-06-7 | | MF: | C13H25NO7 | | MW: | 307.34 | | EINECS: | | | Product Categories: | peg | | Mol File: | 462100-06-7.mol | 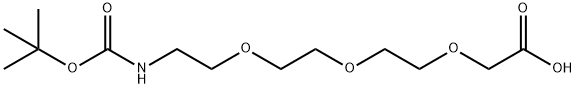 |
| | 2,2-DIMETHYL-4-OXO-3,8,11,14-TETRAOXA-5-AZAHEXADECAN-16-OIC ACID Chemical Properties |
| Boiling point | 460.1±35.0 °C(Predicted) | | density | 1.141±0.06 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | DMF: 5 mg/ml,DMSO: 1 mg/ml,Ethanol: 30 mg/ml | | form | A neat oil | | pka | 3.39±0.10(Predicted) | | color | Colorless to light yellow | | InChI | InChI=1S/C13H25NO7/c1-13(2,3)21-12(17)14-4-5-18-6-7-19-8-9-20-10-11(15)16/h4-10H2,1-3H3,(H,14,17)(H,15,16) | | InChIKey | IFSMYFLQPDFUOI-UHFFFAOYSA-N | | SMILES | C(OC(C)(C)C)(=O)NCCOCCOCCOCC(O)=O | | CAS DataBase Reference | 462100-06-7 |
| WGK Germany | WGK 3 | | HS Code | 2924190090 | | Storage Class | 10 - Combustible liquids |
| | 2,2-DIMETHYL-4-OXO-3,8,11,14-TETRAOXA-5-AZAHEXADECAN-16-OIC ACID Usage And Synthesis |
| Description | t-Boc-N-amido-PEG3-CH2CO2H is a PEG linker containing a terminal carboxylic acid and Boc-protected amino group. The hydrophilic PEG spacer increases solubility in aqueous media. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine. | | Uses | This is a crosslinker with a t-Boc protected amine on one end and a carboxyl group on the other end. The compound contains three PEG units to help improve solubility. | | reaction suitability | reagent type: linker | | Synthesis | To a 1000 mL three-neck flask was added 34.8 g of tert-butyl (2-(2-(2-hydroxyethoxy)ethoxy)ethyl)carbamate (1.0 eq.), 150 mL of toluene, and 150 mL of THF, 58.2 g of bromoacetic acid (3 eq.), and stirred to mix. The reaction mixture was heated to 45?50°C followed by the slow addition of 33.5 g of sodium hydroxide (6 eq.). The reaction was continued at room temperature overnight. The progress of the reaction was monitored by TLC. After completion of the reaction, the solvent was removed by evaporation. The residue was separated by extraction with water and ethyl acetate. The aqueous phase was adjusted to pH 3 with hydrochloric acid and subsequently extracted with dichloromethane. The dichloromethane layers were combined, dried over anhydrous sodium sulfate, filtered and concentrated to give 18 g of 5,8,11-trioxa-2-azatridecanedioic acid 1-tert-butyl ester (BP103a05) as an oil. | | IC 50 | PEGs; Alkyl/ether | | References | [1] Inorganica Chimica Acta, 2011, vol. 365, # 1, p. 38 - 48
[2] Patent: EP3321279, 2018, A1. Location in patent: Paragraph 0066 |
| | 2,2-DIMETHYL-4-OXO-3,8,11,14-TETRAOXA-5-AZAHEXADECAN-16-OIC ACID Preparation Products And Raw materials |
|