- BOC-SAR-OH
-

- $0.00 / 1G/KG
-
2026-02-15
- CAS:13734-36-6
- Min. Order: 1G/KG
- Purity: 99%
- Supply Ability: 10000000KG
- Boc-Sar-OH
-

- $0.00/ kg
-
2026-02-02
- CAS:13734-36-6
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T
- BOC-SAR-OH
-
- $5.00 / 1KG
-
2025-09-25
- CAS:13734-36-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
|
| | BOC-SAR-OH Basic information |
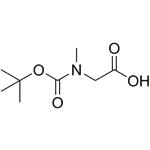
| Product Name: | BOC-SAR-OH | | Synonyms: | BOC-SARCOSINE;BOC-SAR;BOC-SAR-OH;Boc-N-methylglycine, Boc-sarcosine;2-[methyl-[(2-methylpropan-2-yl)oxy-oxomethyl]amino]acetate;N-[(1,1-dimethylethoxy)carbonyl]sarcosine;Glycine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-;Glycine, N-methyl-N-(t-butoxycarbonyl)- | | CAS: | 13734-36-6 | | MF: | C8H15NO4 | | MW: | 189.21 | | EINECS: | 237-306-0 | | Product Categories: | Amino Acids;Sarcosine [Sar];Unusual Amino Acids | | Mol File: | 13734-36-6.mol |  |
| | BOC-SAR-OH Chemical Properties |
| Melting point | 88-90 °C | | Boiling point | 324.46°C (rough estimate) | | density | 1.2321 (rough estimate) | | refractive index | 1.4540 (estimate) | | storage temp. | 2-8°C | | solubility | Chloroform (Sparingly), DMSO (Sparingly), Methanol (Slightly) | | pka | 4.03±0.10(Predicted) | | form | Crystals or Crystalline Powder | | color | White | | BRN | 2046827 | | Stability: | Hygroscopic | | Major Application | peptide synthesis | | InChI | InChI=1S/C8H15NO4/c1-8(2,3)13-7(12)9(4)5-6(10)11/h5H2,1-4H3,(H,10,11) | | InChIKey | YRXIMPFOTQVOHG-UHFFFAOYSA-N | | SMILES | C(O)(=O)CN(C(OC(C)(C)C)=O)C | | CAS DataBase Reference | 13734-36-6(CAS DataBase Reference) | | EPA Substance Registry System | Glycine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl- (13734-36-6) |
| Safety Statements | 24/25 | | WGK Germany | 3 | | F | 21 | | TSCA | TSCA listed | | HazardClass | IRRITANT | | HS Code | 29241990 | | Storage Class | 11 - Combustible Solids |
| | BOC-SAR-OH Usage And Synthesis |
| Chemical Properties | white crystals or crystalline powder | | Uses | Boc-N-methylglycine is used in the preparation of Clavatustide A (C563723) and Clavatustide B (C563725), which have been shown to suppress the proliferation of human hepatocellular carcinoma cells under specific conditions. | | reaction suitability | reaction type: Boc solid-phase peptide synthesis | | Synthesis | Triethylamine (23.5 mL, 168.3 mmol) and di-tert-butyl dicarbonate (12.9 mL, 56.1 mmol) were sequentially added to an aqueous (375 mL) solution of sarcosine (5.0 g, 56.1 mmol), and the reaction mixture was stirred for 6 hours at room temperature. After completion of the reaction, hydrochloric acid solution (320 mL, 1 M aqueous solution) and ethyl acetate were added for acidification and extraction. After separation of the aqueous layer, the aqueous layer was further extracted with ethyl acetate. The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give a colorless oil. The oily substance was crystallized at -15 °C to afford the target product 2-((tert-butoxycarbonyl)(methyl)amino)acetic acid (10.6 g, quantitative yield) as a white solid and a mixture of rotary isomers was observed. | | References | [1] Tetrahedron Letters, 2017, vol. 58, # 7, p. 602 - 605
[2] Journal of the American Chemical Society, 2003, vol. 125, # 35, p. 10664 - 10671
[3] Synthesis, 1996, # 10, p. 1246 - 1258
[4] Patent: WO2005/39506, 2005, A2. Location in patent: Page/Page column 93-94 |
| | BOC-SAR-OH Preparation Products And Raw materials |
|