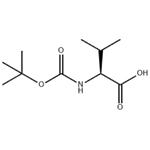
- Boc-L-Val-OH
-
- $0.00/ kg
-
2026-01-22
- CAS:13734-41-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T
- N-Boc-L-valine
-

- $1.00 / 1KG
-
2026-01-22
- CAS:13734-41-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10ton/month
- N-Boc-L-valine
-

- $5.00/ KG
-
2026-01-22
- CAS:13734-41-3
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS
|
| | N-Boc-L-valine Basic information | | Uses |
| | N-Boc-L-valine Chemical Properties |
| Melting point | 77-80 °C(lit.) | | alpha | -6.3 º (c=1,CH3COOH) | | Boiling point | 357.82°C (rough estimate) | | density | 1.1518 (rough estimate) | | vapor pressure | 0.002Pa at 25℃ | | refractive index | -6.5 ° (C=1, AcOH) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | Chloroform, DMF, DMSO, Methanol | | pka | 4.01±0.10(Predicted) | | form | Fine Crystalline Powder | | color | White | | Optical Rotation | [α]20/D 6.2±0.5°, c = 1% in acetic acid | | Water Solubility | insoluble | | BRN | 1711290 | | Major Application | peptide synthesis | | InChI | 1S/C10H19NO4/c1-6(2)7(8(12)13)11-9(14)15-10(3,4)5/h6-7H,1-5H3,(H,11,14)(H,12,13)/t7-/m0/s1 | | InChIKey | SZXBQTSZISFIAO-ZETCQYMHSA-N | | SMILES | CC(C)[C@H](NC(=O)OC(C)(C)C)C(O)=O | | LogP | 1.752 at 25℃ | | CAS DataBase Reference | 13734-41-3(CAS DataBase Reference) | | EPA Substance Registry System | L-Valine, N-[(1,1-dimethylethoxy)carbonyl]- (13734-41-3) |
| Hazard Codes | Xn | | Risk Statements | 20/21/22-36/37/38 | | Safety Statements | 24/25-36-26 | | WGK Germany | 3 | | TSCA | TSCA listed | | HS Code | 2924 19 00 | | HazardClass | IRRITANT | | Storage Class | 11 - Combustible Solids |
| | N-Boc-L-valine Usage And Synthesis |
| Uses | Boc-Vgl-OH is an important organic intermediate to synthetize substituted vinylglycine products.
| | Chemical Properties | N-tert-butoxycarbonyl-L-proline is white fine crystalline powder | | Uses | N-tert-butoxycarbonyl-L-proline is used in amino acid conjugates as proteasome inhibitors. | | Uses | N-Boc-L-valine is used in the multi-peptide synthesis and serves as the amino acid protection monomer. | | reaction suitability | reaction type: Boc solid-phase peptide synthesis
reaction type: C-H Activation
reagent type: ligand
reaction type: Peptide Synthesis |
| | N-Boc-L-valine Preparation Products And Raw materials |
|