- Nickel(II) oxide
-
- $0.00 / 1removed
-
2026-01-12
- CAS:1313-99-1
- Min. Order:
- Purity: 98%
- Supply Ability: 10g
- Nickel(II) oxide
-

- $0.00 / 1KG
-
2025-12-24
- CAS:1313-99-1
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 10000KG
- Nickel(II) oxide
-

- $5.00 / 1kg
-
2025-10-31
- CAS:1313-99-1
- Min. Order: 10kg
- Purity: 99%
- Supply Ability: 600tons
Related articles - Uses of Nickel Monoxide
- Nickel monoxide (synonyms: Nickel(II) oxide, Nickel protoxide or Green nickel oxide) is a green to black colored, inorganic co....
- Oct 10,2019
|
| | Nickel(II) oxide Chemical Properties |
| Melting point | 1960 °C | | bulk density | 0.51g/mL | | density | 6.67 g/mL at 25 °C(lit.) | | storage temp. | Store at room temperature, keep dry and cool | | solubility | insoluble in H2O; soluble in acid solutions | | form | Powder/Solid | | color | green | | Specific Gravity | 6.67 | | Water Solubility | insoluble | | Crystal Structure | NaCl type | | crystal system | Cube | | Merck | 14,6512 | | Space group | Fm3m | | Lattice constant | | a/nm | b/nm | c/nm | α/o | β/o | γ/o | V/nm3 | | 0.418 | 0.418 | 0.418 | 90 | 90 | 90 | 0.073 |
| | Exposure limits | ACGIH: TWA 0.2 mg/m3
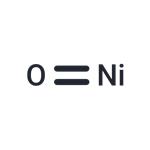
NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 | | Stability: | Stable. Incompatible with strong acids. | | Major Application | battery manufacturing | | InChI | InChI=1S/Ni.O | | InChIKey | GNRSAWUEBMWBQH-UHFFFAOYSA-N | | SMILES | O=[Ni] | | CAS DataBase Reference | 1313-99-1(CAS DataBase Reference) | | EPA Substance Registry System | Nickel(II) oxide (1313-99-1) |
| Hazard Codes | T,Xi | | Risk Statements | 49-43-53-48/23-36/37 | | Safety Statements | 53-45-61-36/37-26 | | RIDADR | 3288 | | WGK Germany | 1 | | RTECS | QR8400000 | | TSCA | TSCA listed | | HS Code | 28254000 | | Storage Class | 6.1C - Combustible acute toxic Cat.3
toxic compounds or compounds which causing chronic effects | | Hazard Classifications | Aquatic Chronic 4
Carc. 1A Inhalation
Skin Sens. 1
STOT RE 1 Inhalation | | Hazardous Substances Data | 1313-99-1(Hazardous Substances Data) |
| | Nickel(II) oxide Usage And Synthesis |
| Physical Properties | Green cubic crystals; transforms to a grayish black octahedral form, known as black oxide, when strongly ignited; black oxide has a metallic luster; density of green oxide is 6.72 g/cm3; Mohs hardness 5.5; melts at 1955°C; insoluble in water; soluble in acids at ordinary temperatures; black form dissolves in hot acids.

| | Occurrence | The oxide occurs in nature in the mineral, bunsenite.
| | Uses | Nickel (II) oxide is used in the ceramic industry for making frit, ferrites, and coloring porcelain. The oxide in sinter form is used in the production of nickel- steel alloys. It supplies oxygen to the melt for removal of carbon as carbon dioxide. Some other important uses of Nickel (II) oxide include preparation of many nickel salts, specialty chemicals, and nickel catalysts. It also is used as an electrode in fuel cells. | | Preparation | Nickel (II) oxide is prepared by heating pure nickel powder with oxygen at a temperature above 400°C. In some commercial processes, green Nickel (II) oxide is made by heating a mixture of nickel powder and water in air at 1,000°C. Adding some Nickel (II) oxide to the above mixture enhances the rate of reaction. An alternative method of preparation of the green oxide involves thermal decomposition of an oxo acid salt of nickel at elevated temperatures. Thus, nickel nitrate, nickel sulfate or, more conveniently, nickel carbonate when heated at 1,000°C, yields the green oxide. The black oxide, on the other hand, is produced at a lower temperature from incomplete calcination of the carbonate or nitrate salt at 600°C. The oxygen content of the black form is slightly greater than its green counterpart. | | Reactions | Several nickel salts are obtained by reactions of nickel oxide with mineral acids. Thus, the reaction of black nickel oxide with hot dilute sulfuric acid forms nickel sulfate, NiSO4•6H2O. Similarly, dilute nitric acid, hydrochloric, and hydrobromic acids when heated react with the black form of nickel oxide to yield corresponding nickel salts as hexahydrates.
Heating nickel oxide with hydrogen, carbon, or carbon monoxide reduces it to metallic nickel.
Nickel oxide combines with sodium or potassium hydroxide at elevated temperatures (>700°C), forming sodium or potassium nickelate; i.e., K2NiO2:
NiO + 2NaOH → Na2NiO2 + H2O
| | Chemical Properties | Nickel(II) oxide is Brownish black or black powder. | | Uses | Nickel salts, porcelain painting, fuel cell electrodes. | | Uses | Nickel(II) oxide is used in the preparation of nickel alloys including nickel steel alloys, manufacture of glass and porcelain paints. Further nickel oxide is the main component in nickel-iron battery (Edison battery), nickel-cadmium rechargeable battery, and also used in ceramic industry for making frits and porcelain glazes. It also acts as a hydrogenation catalyst. NiO/CNTs (nickel oxide/carbon nanotubes) could be a potential cathode catalyst for oxygen reduction reaction (ORR) in microbial fuel cells (MFCs). It is blended with other high purity oxides and used in a variety of semiconductor applications such as thermistors and varistors. | | Uses | Nickel oxide nanowires have been studied for use in magnetic memory devices; as well as in luminescent materials. | | Preparation | Nickel oxide is prepared by heating pure nickel powder with oxygen at a temperature above 400°C. In some commercial processes, green nickel oxide is made by heating a mixture of nickel powder and water in air at 1,000°C. Adding some nickel oxide to the above mixture enhances the rate of reaction. An alternative method of preparation of the green oxide involves thermal decomposition of an oxo acid salt of nickel at elevated temperatures. Thus, nickel nitrate, nickel sulfate or, more conveniently, nickel carbonate when heated at 1,000°C, yields the green oxide. The black oxide, on the other hand, is produced at a lower temperature from incomplete calcination of the carbonate or nitrate salt at 600°C. The oxygen content of the black form is slight-ly greater than its green counterpart. | | Reactions | Nickel(II) oxide is insoluble in water but soluble in acids as long as it has not been
ignited at a high temperature (under these latter conditions it is converted into grey-black
octahedra having a metallic lustre). It reacts reversibly with hydrogen, the reaction
NiO+H2 ? Ni+H20
proceeding from left to right at relatively low temperatures in a stream of hydrogen. | | General Description | Nickel(II) oxide (NiO) is a metal oxide based nanomaterial with a good semiconducting property. Nanosized nickel oxide can be found in a variety of morphologies which include nanoflowers, spheres, wires, and tubes. It exhibits high performance in applications which require charge transfer and charge transport based processes. It can be prepared by a variety of physical and thermal methods such as sol-gel, hydrothermal and solvothermal techniques. | | Hazard | Confirmed carcinogen. | | Flammability and Explosibility | Non flammable | | Safety Profile | Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Poison by intratracheal,
intravenous, and subcutaneous routes.
Mutation data reported. Can react violently
with fluorine, hydrogen peroxide, hydrogen
sulfide, iodine, barium oxide + air. See also
NICKEL COMPOUNDS. | | Toxics Screening Level | The Initial Risk Screening Level (IRSL) and Secondary Risk Screening Level (SRSL) for
nickel and nickel compounds are 0.006 and 0.06 μg/m3 based on an annual averaging time. |
| | Nickel(II) oxide Preparation Products And Raw materials |
|