Sodium bromite manufacturers
- Sodium Bromite
-

- $10.00 / 1kg
-
2025-05-26
- CAS:7486-26-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 10 ton
- Sodium Bromite
-

- $30.00 / 1KG
-
2021-07-24
- CAS:7486-26-2
- Min. Order: 100KG
- Purity: 99%
- Supply Ability: 500
- Sodium bromite
-

- $7.00 / 1KG
-
2020-01-10
- CAS:7486-26-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG
|
| | Sodium bromite Basic information |
| Product Name: | Sodium bromite | | Synonyms: | bromousacid,sodiumsalt;Sodium bromite;Bromous acid, sodium salt (1:1);Einecs 231-290-9;Bromous acid;Sodium bromite ISO 9001:2015 REACH | | CAS: | 7486-26-2 | | MF: | BrH2NaO2 | | MW: | 136.91 | | EINECS: | 231-290-9 | | Product Categories: | | | Mol File: | 7486-26-2.mol | 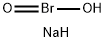 |
| | Sodium bromite Chemical Properties |
| | Sodium bromite Usage And Synthesis |
| Chemical Properties | Pure product is yellow crystal. Industrial products are mostly lemon yellow alkaline solution. Soluble in water. | | Uses | Sodiumbromite is used in preparation method of high-purity sodium bromite aqueous solution. | | Uses | NaBrO2 | | Preparation | Bromous acid can thus be produced by a classical
chemical or an electrochemical method in which hypobromite
is oxidized to the bromite anion:
HBrO+ HClO→HBrO2+ HCl or HBrO+ H2O+ e- →HBrO2+ H2
Also disproportioning of hypobromous acid will give
bromous acid and hydrobromic acid:
2HBrO+ heat→HBrO2+ HBr
In addition, a rearrangement reaction of bromic acid
and hydrobromic acid will produce bromous acid:
2HBrO3+ HBr→3HBrO2 |
| | Sodium bromite Preparation Products And Raw materials |
|