|
|
| | (S)-methyl-2-bromo-3-hydroxypropanoate Basic information |
| Product Name: | (S)-methyl-2-bromo-3-hydroxypropanoate | | Synonyms: | (S)-methyl-2-bromo-3-hydroxypropanoate;2-Bromo-3-hydroxypropanoic acid methyl ester;Methyl 2-bromo-3-hydroxypropionate;2-broMo-3-hydroxybutanoate;Methyl-2-broMo-3-hydroxypropanoate;Propanoic acid, 2-bromo-3-hydroxy-, methyl ester | | CAS: | 7691-28-3 | | MF: | C4H7BrO3 | | MW: | 183 | | EINECS: | | | Product Categories: | | | Mol File: | 7691-28-3.mol | 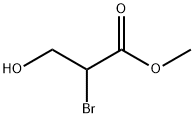 |
| | (S)-methyl-2-bromo-3-hydroxypropanoate Chemical Properties |
| Boiling point | 245℃ | | density | 1.677 | | Fp | 102℃ | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | pka | 13.45±0.10(Predicted) | | Appearance | Colorless to light yellow Liquid | | InChI | InChI=1S/C4H7BrO3/c1-8-4(7)3(5)2-6/h3,6H,2H2,1H3 | | InChIKey | GVXAFLUDYYQGCG-UHFFFAOYSA-N | | SMILES | C(OC)(=O)C(Br)CO |
| | (S)-methyl-2-bromo-3-hydroxypropanoate Usage And Synthesis |
| Synthesis | Methyl 2-bromo-3-hydroxypropionate was synthesized as follows: 2-bromo-3-hydroxypropionic acid (6.0 g, 35 mmol) and a catalytic amount of HBr (0.2 mL, 48% aqueous solution, w/w) were dissolved in methanol (50 mL, 1.2 mol), heated to 50-65 °C, and reacted for 21 hours. Upon completion of the reaction, the excess methanol was removed by rotary evaporation. To the brown liquid residue, dichloromethane (100 mL) was added, and the resulting solution was washed twice each with dilute aqueous NaHCO3 (50 mL) and saturated aqueous NaCl (50 mL) in turn, and then dried with Na2SO4. After filtration, the solvent was removed by rotary evaporation to give methyl 2-bromo-3-hydroxypropionate as a light yellow liquid. The product was purified by silica gel chromatography (eluent: dichloromethane/ether, 90/10, Rf = 0.31) to give a clear liquid product (5.7 g, 87% yield).1H NMR (CDCl3): δ 2.70 (br s, OH), 3.81 (s, CH3), 4.00 (m, CH2OH), 4.35 (t, CHBr).13C NMR (CDCl3): δ 44.2 (CBr), 53.5 (CH3), 63.8 (CH2OH), 169.7 (CO2CH3). Elemental analysis (C, H): calculated values 26.23, 3.86; measured values 25.83, 4.10. | | References | [1] Synlett, 2010, # 13, p. 1947 - 1950
[2] Patent: US8524942, 2013, B2. Location in patent: Page/Page column 17
[3] Journal of Biological Chemistry, 1934, vol. 105, p. 547,551
[4] Patent: US2016/287726, 2016, A1. Location in patent: Paragraph 0081; 0082 |
| | (S)-methyl-2-bromo-3-hydroxypropanoate Preparation Products And Raw materials |
|