|
|
| | (R)-1-Boc-3-cyanopyrrolidine Basic information |
| Product Name: | (R)-1-Boc-3-cyanopyrrolidine | | Synonyms: | (R)-1-Boc-3-cyanopyrrolidine;(R)-1-Boc-3-cyanopyrrolid...;tert-butyl (3R)-3-cyanopyrrolidine-1-carboxylate;(R)-N-Boc-Cyanopyrrolidine;(R)-1-N-Boc-3-Cyano-pyrrolidine(R)-1-N-Boc-3-Cyano-pyrrolidine;(R)-1-Boc-3-cyanopyrrolidine 95+%;1-Pyrrolidinecarboxylic acid, 3-cyano-, 1,1-dimethylethyl ester, (3R)-;tert-butyl (R)-3-cyanopyrrolidine-1-carboxylate | | CAS: | 132945-76-7 | | MF: | C10H16N2O2 | | MW: | 196.25 | | EINECS: | | | Product Categories: | Pyrrole&Pyrrolidine&Pyrroline | | Mol File: | 132945-76-7.mol | 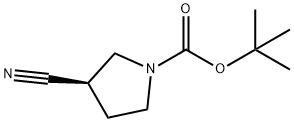 |
| | (R)-1-Boc-3-cyanopyrrolidine Chemical Properties |
| Boiling point | 307.9±35.0 °C(Predicted) | | density | 1.08±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | pka | -3.73±0.40(Predicted) | | form | solid | | Appearance | white solid | | Optical Rotation | [α]/D -21.0±2.0°, c = 1 in methanol | | BRN | 8683216 | | InChI | 1S/C10H16N2O2/c1-10(2,3)14-9(13)12-5-4-8(6-11)7-12/h8H,4-5,7H2,1-3H3/t8-/m0/s1 | | InChIKey | VDDMCMFPUSCJNA-QMMMGPOBSA-N | | SMILES | CC(C)(C)OC(=O)N1CC[C@H](C1)C#N | | CAS DataBase Reference | 132945-76-7(CAS DataBase Reference) |
| Hazard Codes | Xn | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | HS Code | 2933998090 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| | (R)-1-Boc-3-cyanopyrrolidine Usage And Synthesis |
| Uses | (R)-1-Boc-3-cyanopyrrolidine can be used as a key intermediate in the synthesis of a β-proline analog named (3R)-carboxy pyrrolidine. | | Uses | (R)-1-Boc-3-cyanopyrrolidine is used in the preparation of 1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid compounds as anti-microbial agents. | | Synthesis | The general procedure for the synthesis of (R)-1-Boc-3-cyanopyrrolidine from the compound (CAS: 773837-37-9) and S-1-Boc-3-OMS-pyrrolidine is as follows:
b) Synthesis of 1,1-dimethylethyl (3R)-3-cyano-1-pyrrolidine carboxylate
A mixture of 1,1-dimethylethyl (3S)-3-[(methylsulfonyl)oxy]-1-pyrrolidine carboxylic acid ester (211 mmol) with sodium cyanide (633 mmol) in N,N-dimethylformamide (300 mL) was stirred vigorously for 18 h at 100 °C in a nitrogen atmosphere using a mechanical stirrer. After completion of the reaction, the mixture was cooled to room temperature, filtered and washed well with ether. The filtrate was diluted with dilute brine and extracted with ether (4 x 700 mL). The organic extracts were combined, washed with dilute brine, filtered through a pad of sodium sulfate and subsequently concentrated under vacuum. The residue was purified by fast chromatography (eluent: 0-50% ethyl acetate/hexane) to afford the target product (141 mmol, 67% yield).
1H NMR (400 MHz, CDCl3) δ ppm: 1.48 (s, 9H), 2.14-2.37 (m, 2H), 3.00-3.20 (m, 1H), 3.45 (dt, J = 11.05, 6.98 Hz, 1H), 3.53-3.66 (m, 2H), 3.65-3.76 (m, 1H). | | References | [1] Patent: WO2011/56635, 2011, A1. Location in patent: Page/Page column 49-50
[2] Patent: WO2011/66211, 2011, A1. Location in patent: Page/Page column 35
[3] Patent: WO2013/28445, 2013, A1. Location in patent: Page/Page column 51
[4] Patent: WO2012/37298, 2012, A1. Location in patent: Page/Page column 82 |
| | (R)-1-Boc-3-cyanopyrrolidine Preparation Products And Raw materials |
|