- 4-Bromo-2,6-difluoroaniline
-

- $5.00 / 1KG
-
2025-09-25
- CAS:67567-26-4
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: g-kg-tons, free sample is available
|
| | 4-Bromo-2,6-difluoroaniline Basic information |
| Product Name: | 4-Bromo-2,6-difluoroaniline | | Synonyms: | 2,6-DIFLUORO-4-BROMOANILINE;4-BROMO-2,6-DIFLUOROANILINE;4-BROMO-2,6-DIFLUOROANILINE 99%;BUTTPARK 22\07-66;4-Bromo-2,6-difluoroaniline ,98%;4-Bromo-2,6-difluoro;2,6-difluro-4-broMoaniline;(4-BroMo-2,6-difluorophenyl)aMine | | CAS: | 67567-26-4 | | MF: | C6H4BrF2N | | MW: | 208 | | EINECS: | 642-485-7 | | Product Categories: | Anilines, Aromatic Amines and Nitro Compounds;Anilines, Amides & Amines;Bromine Compounds;Fluorine Compounds;Amines;C2 to C6;Nitrogen Compounds | | Mol File: | 67567-26-4.mol | 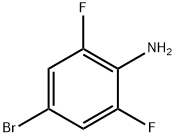 |
| | 4-Bromo-2,6-difluoroaniline Chemical Properties |
| Melting point | 63-65 °C (lit.) | | Boiling point | 188.2±35.0 °C(Predicted) | | density | 1.788±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | pka | 1.11±0.10(Predicted) | | form | Crystalline Powder | | color | Brown | | InChI | InChI=1S/C6H4BrF2N/c7-3-1-4(8)6(10)5(9)2-3/h1-2H,10H2 | | InChIKey | BFQSQUAVMNHOEF-UHFFFAOYSA-N | | SMILES | C1(N)=C(F)C=C(Br)C=C1F | | CAS DataBase Reference | 67567-26-4(CAS DataBase Reference) |
| | 4-Bromo-2,6-difluoroaniline Usage And Synthesis |
| Chemical Properties | Off-white crystalline | | Uses | 4-Bromo-2,6-difluoroaniline was used in synthesis of:
- ammonium 5-bromo-7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate, fluorogenic derivatizating reagent for the determination of aminothiols by HPLC
- olefin tolanes
- 1-(4-amino-3,5-difluoro)-2-(4-butyloxyphenyl)ethyne
- 4′′-n-butyloxy-3,5-difluoroterphenyl-4-amine
- 4-(3-methylbut-1-yn-3-ol)-2,6-difluoroaniline
| | Uses | 4-Bromo-2,6-difluoroaniline is used in the preparation of potent, VEGF receptor tyrosine kinase inhibitors. | | Synthesis | b) Preparation of Intermediate 2: Dissolve 2,6-difluoroaniline (3.0 g, 22.56 mmol) in acetic acid (10 mL). Bromine (1.2 mL) was slowly added dropwise to the above solution. The reaction mixture was stirred at room temperature for 15 minutes. After the reaction was completed, the solvent was evaporated under reduced pressure. The residue was neutralized with aqueous sodium carbonate and the aqueous phase was subsequently extracted with dichloromethane. The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give Intermediate 2 in 92% yield. | | References | [1] Angewandte Chemie - International Edition, 2016, vol. 55, # 4, p. 1544 - 1547
[2] Angew. Chem., 2016, vol. 128, # 4, p. 1569 - 1573,5
[3] Journal of Materials Chemistry C, 2016, vol. 4, # 23, p. 5326 - 5333
[4] Patent: WO2006/15985, 2006, A1. Location in patent: Page/Page column 54
[5] Patent: US6716491, 2004, B2 |
| | 4-Bromo-2,6-difluoroaniline Preparation Products And Raw materials |
|