|
|
| | 4-PYRAZOL-1-YL-BENZONITRILE Basic information |
| Product Name: | 4-PYRAZOL-1-YL-BENZONITRILE | | Synonyms: | 4-PYRAZOL-1-YL-BENZONITRILE;4-(1H-Pyrazol-1-yl);Benzonitrile, 4-(1H-pyrazol-1-yl)-;Benzonitrile, p-pyrazol-1-yl-;4-(1-Pyrazolyl)benzonitrile;4-(1H-PyrazoL;1-(4-Cyanophenyl)pyrazole | | CAS: | 25699-83-6 | | MF: | C10H7N3 | | MW: | 169.18 | | EINECS: | | | Product Categories: | | | Mol File: | 25699-83-6.mol | 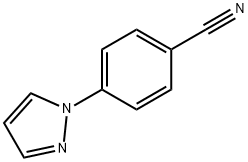 |
| | 4-PYRAZOL-1-YL-BENZONITRILE Chemical Properties |
| Melting point | 89 °C | | Boiling point | 316.5±25.0 °C(Predicted) | | density | 1.13±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | soluble in Methanol | | form | powder to crystal | | pka | -0.63±0.10(Predicted) | | color | White to Light yellow | | InChI | InChI=1S/C10H7N3/c11-8-9-2-4-10(5-3-9)13-7-1-6-12-13/h1-7H | | InChIKey | SLPWCEHHSRUSKN-UHFFFAOYSA-N | | SMILES | C(#N)C1=CC=C(N2C=CC=N2)C=C1 |
| Hazard Codes | Xi | | RIDADR | UN3439 | | HazardClass | 6.1 | | PackingGroup | III | | HS Code | 2933199090 |
| | 4-PYRAZOL-1-YL-BENZONITRILE Usage And Synthesis |
| Synthesis | The general procedure for the synthesis of 4-(1-pyrazolyl)benzonitrile from pyrazole and p-fluorobenzonitrile was as follows: 4-fluorobenzonitrile (204.2 g, 1.0 eq.), pyrazole (138.6 g, 1.22 eq.), and potassium carbonate (281.5 g, 1.22 eq.) were dissolved in DMF (1110 mL), and heated to a temperature of 120°C for 7 hours. After completion of the reaction, the suspension was cooled to 25 °C and diluted with water (2920 mL). The reaction mixture was extracted with methyl tert-butyl ether (MTBE, 3 x 1460 mL), and the organic phases were combined and washed sequentially with water (3 x 1460 mL) and saturated aqueous sodium chloride solution (1460 mL). The organic phase was concentrated at atmospheric pressure until the tank temperature rose to 65 °C, then heptane (1700 mL) was added over 30 min at 60-65 °C and distillation was continued to collect 300 mL of distillate. The solution was stirred at 60-65°C for 15 minutes and subsequently cooled to <5°C. The resulting slurry was filtered, the solid was washed with heptane (2 x 200 mL) and dried under vacuum to constant weight to afford the target product 4-(1-pyrazolyl)benzonitrile as a white solid (245.3 g, 87% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 6.51 (q, 1H), 7.71 (d, 2H), 7.75 (d, 1H), 7.81 (d, 2H), 7.98 (d, 1H). | | References | [1] Patent: WO2009/61271, 2009, A1. Location in patent: Page/Page column 45-46
[2] Bioorganic and Medicinal Chemistry Letters, 2006, vol. 16, # 11, p. 2955 - 2959
[3] Patent: US2011/224229, 2011, A1. Location in patent: Page/Page column 19
[4] Patent: WO2017/87837, 2017, A1. Location in patent: Paragraph 00269
[5] Patent: US9301951, 2016, B2. Location in patent: Page/Page column 231 |
| | 4-PYRAZOL-1-YL-BENZONITRILE Preparation Products And Raw materials |
|