- L-Leu-NCA
-

- $0.00/ kg
-
2025-11-12
- CAS:3190-70-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T+
|
| | (S)-(-)-4-ISOBUTYLOXAZOLIDINE-2,5-DIONE Basic information |
| Product Name: | (S)-(-)-4-ISOBUTYLOXAZOLIDINE-2,5-DIONE | | Synonyms: | (S)-(-)-4-ISOBUTYLOXAZOLIDINE-2,5-DIONE;(S)-4-ISOBUTYL-OXAZOLIDINE-2,5-DIONE;(4S)-4β-(2-Methylpropyl)oxazolidine-2,5-dione;(S)-4β-(2-Methylpropyl)-2,5-oxazolidinedione;L-Leucine NCA;N-Carboxy-L-leucine anhydride;L-Leu-NCA;L-Leucine N-carboxyanhydride | | CAS: | 3190-70-3 | | MF: | C7H11NO3 | | MW: | 157.17 | | EINECS: | 221-692-2 | | Product Categories: | | | Mol File: | 3190-70-3.mol | 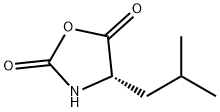 |
| | (S)-(-)-4-ISOBUTYLOXAZOLIDINE-2,5-DIONE Chemical Properties |
| Melting point | 76-77°C | | density | 1.124±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | pka | 9.38±0.40(Predicted) | | Appearance | White to off-white Solid | | Optical Rotation | Consistent with structure |
| Safety Statements | 22-24/25 | | HS Code | 2934999090 |
| Provider | Language |
|
ALFA
| English |
| | (S)-(-)-4-ISOBUTYLOXAZOLIDINE-2,5-DIONE Usage And Synthesis |
| Uses | N-Carboxyleucine Anhydride is a reagent in the synthesis of hybrid polypeptide micelles which are used to load indocyanine green dyes into lab rats for tumor imaging and photothermal effect studies. | | Synthesis | General procedure for the synthesis of (S)-4-isobutyloxazolidine-2,5-dione from phosgene and L-leucine: L-leucine amide (HO-Leu-NH2, 10.0 g, 76.2 mmol) was suspended in 150 mL of anhydrous tetrahydrofuran (THF), and heated to 50 °C. A 20% phosgene solution of toluene (76.0 mL, 152.4 mmol) was slowly added to the amino acid suspension. After about 1 hour, the amino acids were completely dissolved to form a clarified solution. The reaction solution was concentrated on a rotary evaporator, transferred to a beaker, and the product was precipitated by the addition of hexane. The white solid was separated by filtration and dissolved in toluene. The toluene solution was filtered through a bed of diatomaceous earth to remove insoluble impurities. Excess hexane was added to the filtrate to precipitate the product again. N-carboxy-L-leucine anhydride (Leu NCA) was obtained by filtration separation and dried under vacuum. 9.0 g (75% yield) of Leu NCA was finally obtained as a white crystalline solid. Its 1H NMR (d6-DMSO) data were as follows: δ 9.13 (1H), 4.44 (1H), 1.74 (1H), 1.55 (2H), 0.90 (6H) ppm. | | References | [1] Advanced Synthesis and Catalysis, 2004, vol. 346, # 9-10, p. 1247 - 1249
[2] Patent: US2008/274173, 2008, A1. Location in patent: Page/Page column 94
[3] Journal of the Chemical Society, 1950, p. 3222,3225
[4] Patent: US2789973, 1950,
[5] Nippon Kagaku Zasshi, 1956, vol. 77, p. 44,46 |
| | (S)-(-)-4-ISOBUTYLOXAZOLIDINE-2,5-DIONE Preparation Products And Raw materials |
|