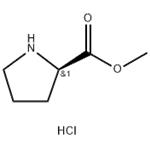
- D-Pro-ome.HCl
-
- $0.00 / 1kg
-
2026-02-02
- CAS:65365-28-8
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T+
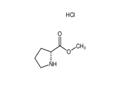
- D-Pro-ome.HCl
-
- $1.00 / 1g
-
2020-01-06
- CAS:65365-28-8
- Min. Order: 1g
- Purity: 98%
- Supply Ability: 100KG
|
| | Methyl pyrrolidine-2-carboxylate hydrochloride Basic information |
| | Methyl pyrrolidine-2-carboxylate hydrochloride Chemical Properties |
| Melting point | 69-71°C | | refractive index | 32 ° (C=1, H2O) | | storage temp. | 2-8°C | | solubility | Methanol (Slightly), Water (Slightly, Sonicated) | | form | Powder or Crystalline Powder | | color | White | | Optical Rotation | [α]/D 33.0±2.0°, c = 1 in H2O | | Sensitive | Hygroscopic | | BRN | 4714125 | | Major Application | peptide synthesis | | InChI | 1S/C6H11NO2.ClH/c1-9-6(8)5-3-2-4-7-5;/h5,7H,2-4H2,1H3;1H/t5-;/m1./s1 | | InChIKey | HQEIPVHJHZTMDP-NUBCRITNSA-N | | SMILES | Cl.COC(=O)[C@H]1CCCN1 | | CAS DataBase Reference | 65365-28-8(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/38-36/37/38 | | Safety Statements | 26-36-28 | | WGK Germany | 3 | | HS Code | 29339900 | | Storage Class | 11 - Combustible Solids |
| Provider | Language |
|
ALFA
| English |
| | Methyl pyrrolidine-2-carboxylate hydrochloride Usage And Synthesis |
| Chemical Properties | Crystalline | | Uses | D-Proline Methyl Ester is a methyl ester of D-Proline (P755990) which is used in the synthesis of (R)-(+)-N-Boc-Pipecolic Acid, (S)-(-)-Coniine, (S)-(+)-Pelletierine, (+)-.beta.-Conhydrine, and (S)-(-)-Ropivacaine and formal synthesis of (-)-Lasubine II and (+)-Cermizine C. | | reaction suitability | reaction type: solution phase peptide synthesis | | Synthesis | The general steps for the synthesis of L-proline methyl ester hydrochloride from methanol and DL-proline are as follows:
Step 1: Synthesis of methylpyrrolidine-2-carboxylic acid hydrochloride
Thionyl chloride (15.7 mL, 217.4 mmol) was slowly added dropwise to a mixed solution of DL-proline (5.0 g, 43.47 mmol) and methanol (50 mL) at about 0 °C, and the dropwise process lasted for about 15 minutes. The reaction mixture was stirred at room temperature for about 16 hours before the solvent was removed by vacuum concentration. The resulting gel was ground with n-pentane, decanted and dried to give D-proline methyl ester hydrochloride as an off-white solid (6.25 g, 87% yield).
The product characterization data were as follows:
1H NMR (400 MHz, DMSO-d6) δ 1.87-2.04 (m, 3H), 2.21-2.28 (m, 1H), 3.22-3.26 (m, 2H), 3.75 (s, 3H), 4.34 (t, J = 7.8 Hz, 1H).
IR (film) υ 3513, 3130, 2597, 1740, 1632, 1452, 1402, 1245, 1178, 1046, 764 cm-1;
MS 130 (M + 1). | | References | [1] Bulletin de la Societe Chimique de France, 1993, vol. 130, p. 584 - 596
[2] Patent: US2010/105755, 2010, A1. Location in patent: Page/Page column 11-12
[3] Molecules, 2008, vol. 13, # 5, p. 1111 - 1119
[4] Journal of Organic Chemistry, 1991, vol. 56, # 8, p. 2775 - 2781
[5] Patent: US2008/76758, 2008, A1. Location in patent: Page/Page column 91; 93 |
| | Methyl pyrrolidine-2-carboxylate hydrochloride Preparation Products And Raw materials |
|