|
|
| | tert-butyl 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1(2H)-carboxylate Basic information |
| Product Name: | tert-butyl 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1(2H)-carboxylate | | Synonyms: | tert-butyl 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1(2H)-carboxylate;3,4-Dihydro-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1(2H)-pyridinecarboxylic acid tert butyl ester;N-Boc-3,4-Dihydropyridine-6-boronic acid pinacol ester;1-(tert-Butoxycarbonyl)-1,4,5,6-tetrahydropyridin-2-ylboronic acid pinacol ester;tert-Butyl 6-(4,4,5,5-tetramethyl-1,3,2-;tert-butyl 6-(tetramethyl-1,3,2-dioxaborolan-2-yl)-1,2,3,4-tetrahydropyridine-1-carboxylate;1-Boc-3,4-dihydropyridine-6-boronic acid pinacol ester;1-Boc-1,4,5,6-tetrahydropyridine-2-boronic Acid Pinacol Ester | | CAS: | 865245-32-5 | | MF: | C16H28BNO4 | | MW: | 309.21 | | EINECS: | | | Product Categories: | | | Mol File: | 865245-32-5.mol | 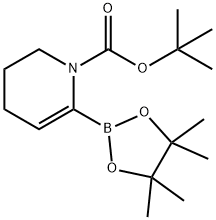 |
| | tert-butyl 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1(2H)-carboxylate Chemical Properties |
| Boiling point | 355.2±52.0 °C(Predicted) | | density | 1.05 | | storage temp. | Storage temp. -20°C | | pka | -0.15±0.40(Predicted) | | Appearance | White to yellow Solid | | InChI | InChI=1S/C16H28BNO4/c1-14(2,3)20-13(19)18-11-9-8-10-12(18)17-21-15(4,5)16(6,7)22-17/h10H,8-9,11H2,1-7H3 | | InChIKey | LEQQJEMLERIVAW-UHFFFAOYSA-N | | SMILES | C1N(C(OC(C)(C)C)=O)C(B2OC(C)(C)C(C)(C)O2)=CCC1 |
| | tert-butyl 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1(2H)-carboxylate Usage And Synthesis |
| Synthesis | General procedure for the synthesis of N-tert-butoxycarbonyl-3,4-dihydropyridine-6-boronic acid pinacol ester from bis(boronic acid) pinacol ester and tert-butyl 6-(((trifluoromethyl)sulfonyl)oxy)-3,4-dihydropyridine-1(2H)-carboxylate: To a solution of tert-butyl 6-(((trifluoromethyl)sulfonyl)oxy)-3,4-dihydropyridine-1(2H)-carboxylate (2.0 g. 6.0 mmol) to a solution of 1,4-dioxane (20 mL) was added pinacol ester of bisboronic acid (2.3 g, 9.0 mmol), potassium carbonate (1.7 g, 12.0 mmol), dichloro-bis(triphenylphosphine)palladium(II) (0.4 g, 0.6 mmol) and triphenylphosphine (0.3 g, 1.2 mmol). The reaction mixture was stirred at 90 °C overnight under nitrogen protection. After completion of the reaction, it was cooled to room temperature, the reaction mixture was diluted with ether and washed with water. The organic phase was dried with anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel fast column chromatography (petroleum ether: ethyl acetate = 10:1) to afford N-tert-butoxycarbonyl-3,4-dihydropyridine-6-boronic acid pinacol ester (0.9 g, 48.2% yield) as a colorless oil.1H NMR (400 MHz, CDCl3) δ 1.26 (12H, s), 1.51 (9H, s), 1.76 (2H , t, J = 6 Hz), 2.02-2.06 (2H, m), 3.43 (2H, t, J = 5.8 Hz), 5.20 (1H, s). | | References | [1] Patent: WO2017/223243, 2017, A1. Location in patent: Paragraph 00330 |
| | tert-butyl 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1(2H)-carboxylate Preparation Products And Raw materials |
|