2,3-DIHYDRO-1,1-DIOXO-1,2-BENZISOTHIAZOLE manufacturers
|
| | 2,3-DIHYDRO-1,1-DIOXO-1,2-BENZISOTHIAZOLE Basic information |
| Product Name: | 2,3-DIHYDRO-1,1-DIOXO-1,2-BENZISOTHIAZOLE | | Synonyms: | 2,3-dihydro-1lambda6,2-benzothiazole-1,1-dione;2,3-DIHYDRO-1,1-DIOXO-1,2-BENZISOTHIAZOLE;1,2-benzoisothiazoline 1,1-dioxide;2,3-dihydro-1,2-benzothiazole 1,1-dioxide;2,3-dihydro-1,2-benzisothiazole 1,1-dioxide;2,3-Dihydrobenzo[d]isothiazole 1,1-dioxide;1,2-Benzisothiazole, 2,3-dihydro-, 1,1-dioxide | | CAS: | 936-16-3 | | MF: | C7H7NO2S | | MW: | 169.2 | | EINECS: | | | Product Categories: | pharmacetical | | Mol File: | 936-16-3.mol | 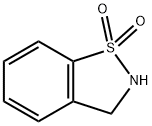 |
| | 2,3-DIHYDRO-1,1-DIOXO-1,2-BENZISOTHIAZOLE Chemical Properties |
| Melting point | 105-107 °C(Solv: water (7732-18-5)) | | Boiling point | 325.8±45.0 °C(Predicted) | | density | 1.396±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | pka | 11.21±0.20(Predicted) | | Appearance | White to off-white Solid |
| | 2,3-DIHYDRO-1,1-DIOXO-1,2-BENZISOTHIAZOLE Usage And Synthesis |
| Synthesis | Step 1. Saccharin (10.0 g, 54.6 mmol) was slowly added to a 300 mL THF solution of LiAlH4 (2.24 g, 59.0 mmol) at 0 °C. The reaction mixture was stirred at 15 °C for 3 h in an inert atmosphere. Upon completion of the reaction, EtOAc (100 mL) was slowly added followed by 10% H2SO4 (100 mL). The organic layer was separated and washed with 100 mL of 5% sodium carbonate solution, dried over anhydrous sodium sulfate, filtered and concentrated to afford 1,2-dihydro-1,1-dioxo-1,2-benzisothiazole (4.4 g, 97%). | | References | [1] Patent: WO2018/49324, 2018, A1. Location in patent: Page/Page column 25
[2] Organic Letters, 2014, vol. 16, # 6, p. 1550 - 1553
[3] Patent: WO2017/21805, 2017, A1. Location in patent: Page/Page column 174
[4] Patent: US2013/303524, 2013, A1. Location in patent: Paragraph 0268-0269
[5] Journal of Medicinal Chemistry, 2016, vol. 59, # 19, p. 8868 - 8878 |
| | 2,3-DIHYDRO-1,1-DIOXO-1,2-BENZISOTHIAZOLE Preparation Products And Raw materials |
|