|
|
| | Methyl 3-bromoindazole-5-carboxylate Basic information |
| Product Name: | Methyl 3-bromoindazole-5-carboxylate | | Synonyms: | Methyl 3-bromoindazole-5-carboxylate;Methyl 3-bromoindazole-5-...;Methyl 3-BroMo-1H-indazole-5-carboxylate;Methyl 3-broMoindazol-5-carboxylate;3-BROMO-1H-INDAZOLE-5-CARBOXYLIC ACID METHYL ESTER;methyl 3-bromo-2H-indazole-5-carboxylate;3-bromo-2H-indazole-4-carboxylic acid methyl ester;1H-Indazole-5-carboxylic acid, 3-bromo-, methyl ester | | CAS: | 1086391-06-1 | | MF: | C9H7BrN2O2 | | MW: | 255.07 | | EINECS: | | | Product Categories: | | | Mol File: | 1086391-06-1.mol | 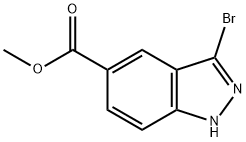 |
| | Methyl 3-bromoindazole-5-carboxylate Chemical Properties |
| Boiling point | 399.7±22.0 °C(Predicted) | | density | 1.709 | | storage temp. | Store at room temperature | | pka | 10.62±0.40(Predicted) | | Appearance | White to yellow Solid |
| | Methyl 3-bromoindazole-5-carboxylate Usage And Synthesis |
| Synthesis | General procedure for the synthesis of methyl 3-bromo-1H-indazole-5-carboxylate from methyl 1H-indazole-5-carboxylate: a mixture of methyl 1H-indazole-5-carboxylate (1.7 g, 0.01 mol) with N-bromosuccinimide (NBS, 2.1 g, 0.012 mol) in tetrahydrofuran (THF, 10 ml) was stirred at room temperature overnight. After completion of the reaction, the mixture was concentrated to give a residue. To the residue was added dichloromethane (DCM, 5 ml), stirred for 30 min and filtered to give methyl 3-bromo-1H-indazole-5-carboxylate (1.9 g, 80% yield) as a light yellow solid. | | References | [1] Patent: US2014/128391, 2014, A1. Location in patent: Paragraph 0392; 0393; 0394
[2] Patent: WO2013/78237, 2013, A1. Location in patent: Page/Page column 54 |
| | Methyl 3-bromoindazole-5-carboxylate Preparation Products And Raw materials |
|