| Company Name: |
Beijing Eternalchem Co,. Ltd.
|
| Tel: |
010-59484199 18611897322 |
| Email: |
sales@eternalchem.com |
| Products Intro: |
Product Name:(Z)-ethyl 2-(hydroxyimino)-2-(pyridin-2-yl)acetate
CAS:154410-82-9
|
| Company Name: |
CEG Chemical Science&Technology Co., Ltd.
|
| Tel: |
Mobile:13665161512 |
| Email: |
|
| Products Intro: |
Product Name:(Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate
CAS:154410-82-9
Purity:>95% Package:5g, 10g, 25g
|
| Company Name: |
Shanghai HuanChuan Industry Co.,Ltd.
|
| Tel: |
021-61478794 |
| Email: |
sales@hcshhai.com |
| Products Intro: |
Product Name:(Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate
CAS:154410-82-9
Purity:95% Package:25g 100g
|
|
| | (Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate Basic information |
| Product Name: | (Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate | | Synonyms: | (Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate;methyl (2Z)-2-nitroso-2-(1H-pyridin-2-ylidene)acetate;ethyl (Z)-2-(hydroxyimino)-2-(pyridin-2-yl)acetate;methyl 2-(N-hydroxyimino)-2-(pyridin-2-yl)acetate;Methyl 2-(hydroxyimino)-2-(pyridin-2-yl)acetate;(Z)-ethyl 2-(hydroxyimino)-2-(pyridin-2-yl)acetate;2-Pyridineacetic acid, α-(hydroxyimino)-, methyl ester;Methyl (Z)-2-(hydroxyimino)-2-(pyridin-2-yl)acetate | | CAS: | 154410-82-9 | | MF: | C8H8N2O3 | | MW: | 180.16 | | EINECS: | | | Product Categories: | | | Mol File: | 154410-82-9.mol | 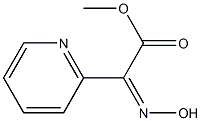 |
| | (Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate Chemical Properties |
| Boiling point | 337.2±34.0 °C(Predicted) | | density | 1.26±0.1 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | pka | 7.75±0.50(Predicted) |
| | (Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate Usage And Synthesis |
| Synthesis | The general procedure for the synthesis of methyl 2-(oxime)-2-(pyridin-2-yl)acetate from methyl 2-pyridylacetate was as follows: to a stirring solution of methyl 2-pyridylacetate (8.9 mL, 0.66 mmol) in acetic acid (16 mL) at 0 °C, an aqueous sodium nitrite solution (4.67 g, 0.66 mmol, 14 mL) was slowly added. After addition, the reaction mixture was continued to be stirred at room temperature for 40 min. Subsequently, water (30 mL) was added and the mixture was stirred for another 1 hour. Upon completion of the reaction, the mixture was concentrated to remove most of the acetic acid and alkalized with aqueous sodium carbonate to pH 8-9. The aqueous phase was extracted with ethyl acetate (3×). The organic phases were combined, dried with magnesium sulfate, filtered, and concentrated under vacuum. Finally, the product was dried in a vacuum oven to afford methyl 2-(oxime)-2-(pyridin-2-yl)acetate (11.6 g, 97% yield) as an off-white solid with a mass spectrum m/z 181.6 [M + 1]+. | | References | [1] Patent: WO2009/70485, 2009, A1. Location in patent: Page/Page column 131
[2] Journal of Heterocyclic Chemistry, 1993, vol. 30, # 5, p. 1253 - 1260
[3] Zhurnal Obshchei Khimii, 1935, vol. 5, p. 1699,1703
[4] Chem. Zentralbl., 1935, vol. 106, # II, p. 3240
[5] Journal of Medicinal Chemistry, 2013, vol. 56, # 20, p. 8049 - 8065 |
| | (Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate Preparation Products And Raw materials |
|