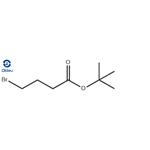
- T-BUTYL 4-BROMOBUTYRATE
-
- $0.00 / 25kg
-
2025-12-01
- CAS:110661-91-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- T-BUTYL 4-BROMOBUTYRATE
-

- $1.00 / 1KG
-
2019-07-06
- CAS:110661-91-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: Customized
|
| | T-BUTYL 4-BROMOBUTYRATE
Basic information |
| Product Name: | T-BUTYL 4-BROMOBUTYRATE
| | Synonyms: | Butanoic acid, 4-broMo-,1,1-diMethylethyl ester;tert-Butyl 4-bromobutanoate, tech;tert-Butyl4-bromobutanoate95%;4-Bromobutanoic acid tert-butyl ester;Elagolix Impurity 32;Elagolix Impurity 12;ert-Butyl4-bromobutanoate;1,1-Dimethylethyl 4-bromobutanoate | | CAS: | 110661-91-1 | | MF: | C8H15BrO2 | | MW: | 223.11 | | EINECS: | | | Product Categories: | Acids & Esters;Bromine Compounds | | Mol File: | 110661-91-1.mol |  |
| | T-BUTYL 4-BROMOBUTYRATE
Chemical Properties |
| Boiling point | 225.9±23.0 °C(Predicted) | | density | 1.258±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C(protect from light) | | solubility | soluble in Chloroform, Hexane | | form | liquid | | color | Colourless to light yellow |
| | T-BUTYL 4-BROMOBUTYRATE
Usage And Synthesis |
| Uses | t-Butyl 4-Bromobutyrate. is a potent CB1 receptor antagonist involved in the cannabinoid-1 receptor signalling process. Inhibition of this receptor has been demonstrated to inhibit feeding behaviors.
Antiinflammatory. | | Synthesis | The general procedure for the synthesis of tert-butyl 4-bromobutyrate from 4-bromobutyric acid and tert-butanol was as follows: 4-bromobutyric acid (2.57 g, 15.39 mmol, 1 eq.) was dissolved in dichloromethane (20 mL), magnesium sulfate (7.74 g, 64.30 mmol, 4.2 eq.), tert-butanol (5.70 g, 76.95 mmol, 5 eq.) and concentrated sulfuric acid (0.25 mL). The reaction mixture was stirred at room temperature for 2 days. Upon completion of the reaction, the reaction was quenched by the addition of saturated aqueous sodium bicarbonate and the reaction mixture was extracted with dichloromethane. The organic layers were combined, washed with saturated sodium chloride solution and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to give the crude product. The crude product was purified by medium pressure silica gel column chromatography (eluent: n-hexane/ethyl acetate=50/50) to afford the target compound tert-butyl 4-bromobutyrate (1.17 g, 5.24 mmol, 47% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3): 1H NMR δ 1.27 (s, 9H), 1.91-1.98 (m, 2H), 2.22 (t, 2H, J=7.2Hz), 3.27 (t, 2H, J=6.5Hz); 13C NMR δ 27.9 , 28.0, 32.8, 33.7, 80.5, 171.7. | | References | [1] Tetrahedron Asymmetry, 2004, vol. 15, # 8, p. 1247 - 1258
[2] ACS Combinatorial Science, 2016, vol. 18, # 12, p. 710 - 722
[3] Angewandte Chemie - International Edition, 2010, vol. 49, # 15, p. 2738 - 2742
[4] European Journal of Organic Chemistry, 2003, # 8, p. 1486 - 1493
[5] Patent: US2018/52109, 2018, A1. Location in patent: Paragraph 0156; 0157; 0158; 0159 |
| | T-BUTYL 4-BROMOBUTYRATE
Preparation Products And Raw materials |
|