Asciminib hydrochloride manufacturers
|
| | Asciminib hydrochloride Basic information |
| Product Name: | Asciminib hydrochloride | | Synonyms: | Asciminib hydrochloride;N-(4-(chlorodifluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1H-pyrazol-5-yl)nicotinamide hydrochloride;Asciminib HCl;(R)-N-(4-(chlorodifluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1H-pyrazol-3-yl)nicotinamide hydrochloride;Asciminib hydrochloride
(ABL-001);(R)-N-(4-(Chlorodifluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1H-pyrazol-5-yl)nicotinamide hydrochloride;(R)-N-(4-(Chloro-difluoromethoxy)phenyl)-6-(3-hydroxy-pyrrolidin-1-yl)-5-(1H-pyrazol-3-yl)-pyridin-3-carboxamide HCl;N-[4-[chloro(difluoro)methoxy]phenyl]-6-[(3R)-3-hydroxypyrrolidin-1-yl]-5-(1H-pyrazol-5-yl)pyridine-3-carboxamide hydrochloride | | CAS: | 2119669-71-3 | | MF: | C20H18ClF2N5O3. HCl | | MW: | 486.3 | | EINECS: | | | Product Categories: | API | | Mol File: | 2119669-71-3.mol | 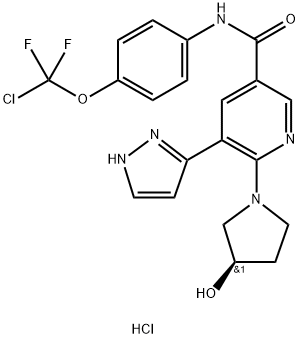 |
| | Asciminib hydrochloride Chemical Properties |
| storage temp. | 4°C, away from moisture | | form | Solid | | color | White to off-white | | InChIKey | HGCOOPLEWPBLOY-XOIICWPPNA-N | | SMILES | C(C1C=NC(N2C[C@H](O)CC2)=C(C2=NNC=C2)C=1)(=O)NC1C=CC(OC(F)(F)Cl)=CC=1.Cl |&1:7,r| |
| | Asciminib hydrochloride Usage And Synthesis |
| Description | Asciminib Hydrochloride is the hydrochloride form of asciminib, an orally bioavailable variant Bcr-Abl1 tyrosine kinase inhibitor with antitumour activity. Upon administration, aximinib targets and binds to the myristoyl pocket of the Bcr-Abl1 fusion protein that is distinct from the ATP-binding domain, thereby inhibiting the activity of wild-type Bcr-Abl and certain mutants, including the T315I mutation. This binding inhibits Bcr-Abl1-mediated proliferation and enhances apoptosis in Philadelphia chromosome-positive (Ph+) haematological malignancies.The Bcr-Abl1 fusion protein tyrosine kinase is an aberrant enzyme produced by Philadelphia chromosome-containing leukaemia cells. | | Uses | Asciminib (ABL001) hydrochloride is a potent and selective allosteric BCR-ABL1 inhibitor, which inhibits Ba/F3 cells grown with an IC50 of 0.25 nM[1]. | | Indications | Asciminib hydrochloride is approved for the treatment of adult patients with chronic myeloid leukaemia (CML) who are in the chronic phase and are Philadelphia chromosome positive. This includes:
Patients who have been treated with at least two tyrosine kinase inhibitors;
Patients with CML who have the T315I mutation;
Newly diagnosed CML patients | | in vivo | Single doses of 7.5, 15 and 30 mg/kg Asciminib, administered to mice bearing KCL- 22 xenografts, inhibits pSTAT5 (Tyr694), which return to baseline at 10, 12 and 16-20 h after administration of the dose, respectively. In mice implanted with KCL-22 tumors, the minimum dose of Asciminib required for complete regression is 7.5 mg/kg twice a day (BID) or 30 mg/kg once a day (QD), and is tolerated at doses up to 250 mg/kg BID[1]. | | References | [1] Wylie AA, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017 Mar 30;543(7647):733-737. DOI:10.1038/nature21702 |
| | Asciminib hydrochloride Preparation Products And Raw materials |
|