Product Details
| Product Name: Abemaciclib Nitroso Impurity 43 | CAS No.: 1231930-57-6 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C25H28F2N8 |
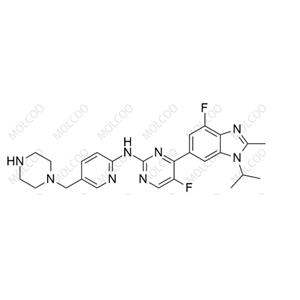
Abemaciclib Nitroso Impurity 43

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: A066043
English Name: Abemaciclib Impurity 43
English Alias: 5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)-N-(5-(piperazin-1-ylmethyl)pyridin-2-yl)pyrimidin-2-amine
CAS Number: 1231930-57-6
Molecular Formula: C₂₅H₂₈F₂N₈
Molecular Weight: 478.54
Advantages
Well-defined and distinct structure: Contains fluorinated benzimidazole, pyrimidine, and piperazinylmethylpyridine moieties, with structural differences from abemaciclib in substituent positions or types. It can be accurately identified by techniques such as HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: The conjugated system of polycyclic aromatic structures and multiple amino groups is highly stable. As a by-product of incomplete coupling reactions in abemaciclib synthesis, it directly reflects the efficiency of key steps, improving process tracing accuracy;
High detection sensitivity: The electronegativity of fluorine atoms and strong UV absorption (260-280nm) of the polycyclic conjugated system, combined with characteristic mass response (m/z 479 [M+H]⁺), enable trace analysis via LC-MS, compatible with pyrimidine-based CDK4/6 inhibitor detection systems.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Abemaciclib Impurity 43 in abemaciclib APIs and formulations, ensuring residual impurities meet quality standards;
Synthesis process optimization: Optimizing coupling reaction conditions (e.g., catalyst selection, temperature) by monitoring impurity content to enhance target product selectivity and reduce by-product formation;
Impurity profile enrichment: Contributing to the completeness of abemaciclib’s impurity profile, providing key data for comprehensive purity assessment and supporting drug registration applications.
Background Description
Research Status
Analytical method validation: Developing UPLC-MS/MS assays for simultaneous quantification of abemaciclib and its impurity 43, achieving detection limits as low as 0.1 ppb for pharmaceutical analysis;
Synthetic efficiency enhancement: Designing novel coupling strategies to minimize impurity formation by improving regioselectivity in benzimidazole-pyrimidine conjugation;
Toxicological screening: Evaluating potential cytotoxicity of the impurity via in vitro cell assays to establish scientifically based impurity limits;
Formulation compatibility studies: Assessing interactions between the impurity and excipients to ensure stability of abemaciclib formulations during shelf life.
Product Information
Product Number: A066043
English Name: Abemaciclib Impurity 43
English Alias: 5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)-N-(5-(piperazin-1-ylmethyl)pyridin-2-yl)pyrimidin-2-amine
CAS Number: 1231930-57-6
Molecular Formula: C₂₅H₂₈F₂N₈
Molecular Weight: 478.54
Advantages
Well-defined and distinct structure: Contains fluorinated benzimidazole, pyrimidine, and piperazinylmethylpyridine moieties, with structural differences from abemaciclib in substituent positions or types. It can be accurately identified by techniques such as HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: The conjugated system of polycyclic aromatic structures and multiple amino groups is highly stable. As a by-product of incomplete coupling reactions in abemaciclib synthesis, it directly reflects the efficiency of key steps, improving process tracing accuracy;
High detection sensitivity: The electronegativity of fluorine atoms and strong UV absorption (260-280nm) of the polycyclic conjugated system, combined with characteristic mass response (m/z 479 [M+H]⁺), enable trace analysis via LC-MS, compatible with pyrimidine-based CDK4/6 inhibitor detection systems.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Abemaciclib Impurity 43 in abemaciclib APIs and formulations, ensuring residual impurities meet quality standards;
Synthesis process optimization: Optimizing coupling reaction conditions (e.g., catalyst selection, temperature) by monitoring impurity content to enhance target product selectivity and reduce by-product formation;
Impurity profile enrichment: Contributing to the completeness of abemaciclib’s impurity profile, providing key data for comprehensive purity assessment and supporting drug registration applications.
Background Description
Research Status
Analytical method validation: Developing UPLC-MS/MS assays for simultaneous quantification of abemaciclib and its impurity 43, achieving detection limits as low as 0.1 ppb for pharmaceutical analysis;
Synthetic efficiency enhancement: Designing novel coupling strategies to minimize impurity formation by improving regioselectivity in benzimidazole-pyrimidine conjugation;
Toxicological screening: Evaluating potential cytotoxicity of the impurity via in vitro cell assays to establish scientifically based impurity limits;
Formulation compatibility studies: Assessing interactions between the impurity and excipients to ensure stability of abemaciclib formulations during shelf life.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $1.00/1kg |
VIP2Y
|
S&Y Biochem Co.,Ltd
|
2024-10-14 | |
| $1.10/100kg |
VIP1Y
|
ZHENGZHOU JIUYI TIME NEW MATERIALS CO,.LTD
|
2025-08-14 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-02-06 |










 China
China