| Identification | More | [Name]
2-CHLORO-1,3-BUTADIENE | [CAS]
126-99-8 | [Synonyms]
2-CHLORO-1,3-BUTADIENE
B-CHLOROPRENE
CHLOROPRENE
1,3-Butadiene, 2-chloro-
1,3-Chloro-2-butadiene
2-Chloor-1,3-butadieen
2-Chlor-1,3-butadien
2-chloro-1,3-butadiene(chloroprene)
2-chloro-1,3-butadiene(chloroprene)(50%inxylene)
2-chloro-3-butadiene
2-chloro-buta-1,3-diene
2-Chlorobuta-1,3-diene
2-Chlorobutadiene
2-Chlorobutadiene 1,3
2-chlorobutadiene-1,3
2-Chloroprene
2-Cloro-1,3-butadiene
3-Butadiene,2-chloro-1
alpha-Chloroprene
Baypren 110 | [EINECS(EC#)]
204-818-0 | [Molecular Formula]
C4H5Cl | [MDL Number]
MFCD00014928 | [Molecular Weight]
88.54 | [MOL File]
126-99-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Chloroprene is a colorless, flammable liquid possessing a pungent odor. The Odor Threshold is 0.4 milligram per cubic meter | [Melting point ]
-130°C | [Boiling point ]
59.45°C | [density ]
0,958 g/cm3 | [vapor pressure ]
118 at 10 °C, 200 at 20 °C, 275 at 30 °C (quoted, Verschueren, 1983) | [refractive index ]
1.4583 | [Fp ]
11 °C | [storage temp. ]
2-8°C | [solubility ]
Soluble in acetone, benzene, and ether (Weast, 1986) | [form ]
Colorless liquid | [Water Solubility ]
256mg/L at 20℃ | [Henry's Law Constant]
3.20 x 10-2 atm?m3/mol using method of Hine and Mookerjee (1975) | [Exposure limits]
Potential occupational carcinogen. NIOSH REL: 15-min ceiling 1 ppm (3.6
mg/m3), IDLH 300 ppm; OSHA PEL: TWA 25 ppm (90 mg/m3); ACGIH TLV: TWA 10 ppm
(adopted). | [LogP]
2.525 at 20℃ | [Uses]
Manufacture of neoprene. | [CAS DataBase Reference]
126-99-8(CAS DataBase Reference) | [IARC]
2B (Vol. Sup 7, 71) 1999 | [EPA Substance Registry System]
Chloroprene (126-99-8) |
| Safety Data | Back Directory | [Hazard Codes ]
F,T | [Risk Statements ]
R10:Flammable.
R20/22:Harmful by inhalation and if swallowed .
R36:Irritating to the eyes.
R48/20:Harmful: danger of serious damage to health by prolonged exposure through inhalation .
R36/37/38:Irritating to eyes, respiratory system and skin .
R11:Highly Flammable.
R45:May cause cancer.
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S53:Avoid exposure-obtain special instruction before use .
S36/37:Wear suitable protective clothing and gloves .
S7:Keep container tightly closed . | [RIDADR ]
1993 | [WGK Germany ]
3 | [HazardClass ]
3.1 | [PackingGroup ]
I | [Safety Profile]
Confirmed carcinogen.
Poison by ingestion, intravenous, and
subcutaneous routes. Moderately toxic by
inhalation. An experimental teratogen.
Experimental reproductive effects. Human
mutation data reported. Human exposure
has caused dermatitis, conjunctivitis,
corneal necrosis, anemia, temporary loss of
hair, nervousness, and irritabhty. Exposure
to the vapor can cause respiratory tract
irritation leading to asphyxia. Other effects
are central nervous system depression, drop
in blood pressure, severe degenerative
changes in the liver, kidneys, lungs, and
other vital organs. A very dangerous fire
hazard when exposed to heat or flame.
Explosive in the form of vapor when
exposed to heat or flame. To fight fire, use
alcohol foam. Auto-oxidlzes in air to form
an unstable peroxide that catalyzes
exothermic polymerization of the monomer.
Incompatible with liquid or gaseous
fluorine. When heated to decomposition it
emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS,
ALIPHATIC . | [Hazardous Substances Data]
126-99-8(Hazardous Substances Data) | [Toxicity]
Acute oral LD50 for mice 260 mg/kg, rats 900 mg/kg (quoted, RTECS, 1985). | [IDLA]
300 ppm |
| Hazard Information | Back Directory | [General Description]
A clear colorless liquid. Flash point-4°F. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a container, the container may rupture violently. Less dense than water. Vapors heavier than air. Used to make neoprene rubber. | [Reactivity Profile]
CHLOROPRENE emits highly toxic fumes of chlorine gas when heated to decomposition. Autooxidizes very rapidly with air and, even at 0°C, produces unstable peroxides that catalyze exothermic polymerization [Bretherick, 5th ed., 1995, p. 507]. This reactivity is greatly slowed by presence of an inhibitor. | [Air & Water Reactions]
Highly flammable. Slightly soluble in water. | [Hazard]
Toxic by ingestion, inhalation, and skin
absorption. Flammable, dangerous fire risk, explosive limits in air 4.0–20%. Eye and upper respiratory
tract irritant. Possible carcinogen. | [Health Hazard]
INHALATION: Fatigue, psychic changes, irritability, oppression in chest, occasionally substernal pain, tachycardia upon exertion. EYES: Can cause conjunctivitis, corneal necrosis and edema of eyelids. SKIN: May cause dermatitis and temporary loss of hair. Rapidly absorbed by skin. | [Potential Exposure]
The major use of chloroprene is in the production of artificial rubber (Neoprene, duprene); polychloroprene elastomers. Chloroprene is extremely reactive, e.g., it can polymerize spontaneously at room temperatures; the process being catalyzed by light, peroxides, and other free radical initiators. It can also react with oxygen to form polymeric peroxides and because of its instability, flammability, and toxicity, chloroprene has no end-product uses as such. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. | [Shipping]
UN1991 Chloroprene, stabilized, Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous material. | [Incompatibilities]
Can form unstable peroxides; chloroprene may polymerize on standing with fire or explosion hazard. May form explosive mixture with air. Reacts with liquid or gaseous fluorine, alkali metals; metal powders, oxidizers, creating a fire or explosion hazard. Attacks some plastics, rubber, and coatings. May accumulate static electrical charges, and may cause ignition of its vapors. | [Description]
Chloroprene, 2-chloro-1,3-butadiene, is a colorless, volatile
synthetic liquid that has a pungent ether-like odor. Synthesis
of chloroprene was first reported by chemists of the E. I. du
Pont de Nemours Company in 1931 following studies of
acetylene polymerization with the objective of producing
synthetic rubber. The chloroprene monomer differs from
isoprene, the fundamental monomer of natural rubber, only
by substitution of chlorine for the methyl group of isoprene.
Chloroprene was observed to polymerize much more
quickly than did isoprene. In industrial processes prior to
1960, chloroprene was produced in relatively high yields by
reacting vinyl acetylene with hydrogen chloride. Today,
chloroprene is produced more efficiently by chlorination of
1,3-butadiene.
When compared with natural rubber the chloroprene
synthetic polymer, polychloroprene, was noted to be much
denser, more resistant to water and hydrocarbon solvents,
less permeable to many gases, and was more resistant
to degradation by oxygen, ozone, hydrogen chloride,
hydrogen fluoride, and other chemicals. Due to desirable
physical and chemical properties, polychloroprene and its
latex polymers are produced in quantities exceeding
200 000 metric tons at a limited number of facilities
around the world. Chloroprene production is closely tied to
demand for polychloroprene. | [Chemical Properties]
Chloroprene (2-chloro-1,3-butadiene) is a flammable, colorless liquid at room temperature with a characteristic ether-like odor. The Odor Threshold is 0.4 milligram per cubic meter. It is slightly soluble in water and more soluble in organic solvents. It has not been found to occur naturally. Chloroprene is very unstable and reacts in air with oxygen and other compounds to form epoxides, peroxides, and other hazardous compounds. | [Waste Disposal]
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. | [Physical properties]
Clear, colorless liquid with a pungent, ether-like odor. The odor threshold is 0.40 mg/m3 (CHRIS,
1984). | [Definition]
A colorless liquid derivative of
butadiene used in the manufacture of neoprene
rubber. | [Preparation]
Preparation of chloroprene from butadiene
In this method, the following route is used:
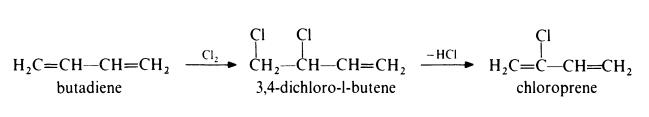
In the first step, butadiene is chlorinated in the vapour phase at 330-420??
and atmospheric pressure. The main products are 3,4-dichloro-l-butene and
1,4-dichloro-2-butene in approximately equal amounts. The latter material is
then isomerized to the former by heating with a copper catalyst such as
cuprous chloride. The 3,4-dichloro-l-butene is dehydrochlorinated by treat�ment with 10% aqueous sodium hydroxide at 85??. Chloroprene is isolated
by distillation under reduced pressure in the presence of polymerization
inhibitors. | [Production Methods]
Chloroprene can be synthesized by addition of HCl to vinyl acetylene H2C=CH?C≡CH +HCl → H2C=CH?CCl=CH2, and by dehydrochlorination of dichlorobutenes or 2,2,3-trichlorobutane. | [Carcinogenicity]
Chloroprene is reasonably anticipated to be a human carcinogen based on evidence of carcinogenicity from studies in experimental
animals. | [Environmental Fate]
Chemical/Physical. Anticipated products from the reaction of chloroprene with ozone or OH
radicals in the atmosphere are formaldehyde, 2-chloroacrolein, OHCCHO, ClCOCHO,
H2CCHCClO, chlorohydroxy acids, and aldehydes (Cupitt, 1980).
Chloroprene will polymerize at room temperature unless inhibited with antioxidants (NIOSH,
1997). Chloroprene is resistant to hydrolysis under neutral and alkaline conditions (Carothers et
al., 1931).
Chloroprene is subject to hydrolysis forming 3-hydroxypropene and HCl. The reported
hydrolysis half-life at 25 °C and pH 7 is 40 yr (Kollig, 1993). | [storage]
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with chloroprene you should be trained onits proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist.Chloroprene must be stored to avoid contact with peroxidesand other oxidizers, such as permanganates, nitrates, chlorates, and perchlorates, since violent reactions occur. Storein tightly closed containers in a cool, well-ventilated area attemperatures below 10℃/50�F. Sources of ignition, such assmoking and open flames, are prohibited where chloropreneis handled, used, or stored. Metal containers involving thetransfer of 5 gallons or more of chloroprene should begrounded and bonded. Drums must be equipped with selfclosing valves, pressure vacuum bungs, and flame arresters.
Use only nonsparking tools and equipment, especially whenopening and closing containers of chloroprene. A regulated,marked area should be established where this chemical ishandled, used, or stored in compliance with OSHAStandard 1910.1045. | [Toxicity evaluation]
Chloroprene is not generally available from chemical
supply firms. Concentrated chloroprene is extremely reactive
unless stored cold, under inert gas, and in presence of oxidation
inhibitors and free radical scavengers. With pure chloroprene
multiple undesirable reactions can occur, including spontaneous
dimerization, polymerization, oxidation, epoxide
formation, and nitration. Due to its reactivity, handling and
transportation of chloroprene are carefully regulated; shipment
of uninhibited chloroprene is forbidden by statute.
Chloroprene is not known to occur naturally. It is not widely
distributed in the environment due its reactivity and its use at
a limited number of facilitiesworldwide. Industrial productionof
chloroprene is accomplished in sealed reactor systems with very
limited fugitiveemissions.Polymerizationprocesses are designed
to be sealed, but must be opened to remove and manipulate
formed polymer. Such opening causes most environmental
release of chloroprene, the majority of which enters the atmosphere.
From National Library of Medicine Toxics Release
Inventory 2010 data, more than 270 000 pounds of chloroprene
was released into the environment. Of that amount more than
97% was released into air at one site in Louisiana, USA. |
|
| Company Name: |
Spectrum Chemical Manufacturing Corp.
|
| Tel: |
021-021-021-67601398-809-809-809 15221380277 |
| Website: |
www.spectrumchemical.com/oa_html/index.jsp?minisite=10020&respid=22372&language=us |
|