| Identification | More | [Name]
Dacarbazine | [CAS]
4342-03-4 | [Synonyms]
4-(3,3-dimethyltriazeno)imidazole-5-carboxamide
4-(dimethyltriazeno)imidazole-5-carboxamide
5-(3,3'-DIMETHYL-1-TRIAZENO)IMIDAZOLE-4-CARBOXAMIDE
5-(3,3-DIMETHYL-1-TRIAZENYL)-1H-IMIDAZOLE-4-CARBOXAMIDE
5-[3,3-DIMETHYL-1-TRIAZENYL]IMIDAZOLE-4-CARBOXAMIDE
DACARBAZINE
DIC
DTIC
NSC-45388
TOSLAB 25071
(dimethyltriazeno)imidazolecarboxamide
4-(3,3-dimethyl-1-triazeno)imidazole-5-carboxamide
4-(5)-(3,3-dimethyl-1-triazeno)imidazole-5(4)-carboxamide
5-(3,3-dimethyl-1-triazeno)-imidazole-4-carboxamid
5-(3,3-dimethyl-1-triazeno1-imidazole-4-carboxamid
5-(3,3-dimethyl-1-triazenyl)-1h-imidazole-4-carboxamid
5-(3,3-dimethyltriazeno)imidazole-4-carboxamide
5-(dimethyltriazeno)imidazole-4-carboxamide
biocarbaziner
deticene | [EINECS(EC#)]
224-396-1 | [Molecular Formula]
C6H10N6O | [MDL Number]
MFCD00057167 | [Molecular Weight]
182.18 | [MOL File]
4342-03-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Dacarbazine is a white to ivory-colored
crystalline solid. | [Melting point ]
199-205°C | [Boiling point ]
315.57°C (rough estimate) | [density ]
1.3206 (rough estimate) | [refractive index ]
1.7500 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Slightly soluble in water and in anhydrous ethanol, practically insoluble in methylene chloride. | [form ]
powder | [pka]
pKa 4.42 (Uncertain) | [color ]
Off-White to Light Yellow | [Water Solubility ]
Slightly soluble in water, DMSO and ethanol
/n | [Merck ]
2798 | [LogP]
-0.240 | [CAS DataBase Reference]
4342-03-4(CAS DataBase Reference) | [IARC]
2B (Vol. 26, Sup 7) 1987 | [EPA Substance Registry System]
4342-03-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi,Xn | [Risk Statements ]
R45:May cause cancer.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S37/39:Wear suitable gloves and eye/face protection .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
NI3950000
| [HS Code ]
29349990 | [Safety Profile]
Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Poison by intraperitoneal
and parenteral routes. Moderately toxic by
ingestion and intravenous routes.
Experimental teratogenic effects. Human
systemic effects by intravenous route:
nausea or vomiting, leukopenia (reduced
white blood cell count), and changes in
dehydrogenase enzymatic activity. Mutation
data reported. When heated to
decomposition it emits toxic fumes of NOx. | [Hazardous Substances Data]
4342-03-4(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
White to ivory microcrystals or off-white crystalline solid. | [Reactivity Profile]
DACARBAZINE(4342-03-4) decomposes explosively at its melting point (250°C). Decomposes in the presence of light. Sensitive to oxidation. | [Air & Water Reactions]
Insoluble in water. | [Potential Exposure]
Dacarbazine is used in cancer chemo-
therapy. Dacarbazine is used as an antineoplastic agent in
the treatment of certain skin cancers, and is occasionally
used in the therapy of other neoplastic diseases which have
become resistant to alternative treatment.br Health professionals who handle this drug (for example,
pharmacists, nurses, and physicians) may possibly be
exposed during drug preparation, administration, or cleanup;
however, the risks can be avoided through use of appropriate
containment equipment and work practices
.People
receiving dacarbazine in treatment are also exposed. | [Fire Hazard]
Flash point data for this chemical are not available. DACARBAZINE is probably combustible. | [First aid]
Skin Contact
: Flood all areas of body that have
contacted the substance with water. Don’t wait to remove
contaminated clothing; do it under the water stream. Use
soap to help assure removal. Isolate contaminated clothing
when removed to prevent contact by others. Eye Contact:
Remove any contact lenses at once. Flush eyes well with
copious quantities of water or normal saline for at least
20 30 minutes. Seek medical attention. Inhalation: Leave
contaminated area immediately; breathe fresh air. Proper
respiratory protection must be supplied to any rescuers. If
coughing, difficult breathing or any other symptoms develop,
seek medical attention at once, even if symptoms develop
many hours after exposure. Ingestion: If convulsions are not
present, give a glass or two of water or milk to dilute the substance. Assure that the person’s airway is unobstructed
and contact a hospital or poison center immediately for
advice on whether or not to induce vomiting. | [Shipping]
UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
ncompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo-
sions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, and epoxides. Explosive decom-
position reported @ 250℃
255℃
| [Description]
Dacarbazine is nevertheless considered the first representative of the series of triazene
derivatives. It has been shown that it is an alkylating agent, and thus this drug inhibits RNA
and protein synthesis to a greater degree than DNA. Dacarbazine is used intravenously for
Hodgkin’s disease, soft-tissue sarcoma, and metastatic melanoma. A synonym of this drug
is diticene. | [Waste Disposal]
It is inappropriate and possi-
bly dangerous to the environment to dispose of expired or
waste drugs and pharmaceuticals by flushing them down
the toilet or discarding them to the trash. Household quanti-
ties of expired or waste pharmaceuticals may be mixed
with wet cat litter or coffee grounds, double-bagged in
plastic, discard in trash. Larger quantities shall carefully
take into consideration applicable DEA, EPA, and FDA
regulations. If possible return the pharmaceutical to the
manufacturer for proper disposal being careful to properly
label and securely package the material. Alternatively, the
waste pharmaceutical shall be labeled, securely packaged
and transported by a state licensed medical waste contractor
to dispose by burial in a licensed hazardous or toxic waste
landfill or incinerator. | [Definition]
ChEBI: A monocarboxylic acid amide that is 1H-imidazole-4-carboxamide which is substituted at position 5 by a 3,3-dimethyltriaz-1-en-1-yl group. It is used for the treatment of metastatic malignant melanoma, and in combination with other drugs
or the treatment of Hodgkin's disease and soft-tissue sarcoma. | [Indications]
Dacarbazine (DTIC-Dome) is activated by photodecomposition
and by enzymatic N-demethylation.
Eventual formation of a methyl carbonium ion results
in methylation of DNA and RNA and inhibition of nucleic
acid and protein synthesis. As with other alkylating
agents, cells in all phases of the cell cycle are susceptible
to dacarbazine.
The plasma half-life of dacarbazine is biphasic, with
a distribution phase of 19 minutes and an elimination
phase of 5 hours. The drug is not appreciably protein
bound, and it does not enter the central nervous system
(CNS). Urinary excretion of unchanged drug is by renal
tubular secretion. Dacarbazine metabolism and decomposition
is complex.
Dacarbazine is the most active agent used in metastatic
melanoma, producing a 20% remission rate. It is also
combined with doxorubicin and other agents in the treatment
of various sarcomas and Hodgkin’s disease.
Dacarbazine may cause severe nausea and vomiting.
Leukopenia and thrombocytopenia occur 2 weeks after
treatment, with recovery by 3 to 4 weeks. Less common
is a flulike syndrome of fever, myalgias, and malaise.
Alopecia and transient abnormalities in renal and hepatic
function also have been reported. | [Biochem/physiol Actions]
Dacarbazine is a purine analog of naturally occurring purine precursor 5-amino-1H-imidazole-4-carboxamide (AIC). It is a synthetic triazine antineoplastic agent that exerts cytotoxic effects by acting as an alkylating agent and by inhibiting DNA synthesis and inducing apoptosis. It is known to induce hepatotoxicity in mice. Dacarbazine is not cell cycle-phase specific. | [Clinical Use]
This DNA methylating agent is administered IV as a single agent in the treatment of malignant melanoma and in combination with other agents in the treatment of metastatic melanoma. | [Synthesis]
Dacarbazine, 5-(3,3-dimethyl-1-triazeno)imidazol-4-carboxamide (30.6.5),
is made by diazotation of 5-aminoimidazol-4-carboxamide with nitrous acid, which results
in the formation of 5-diazoimidazol-4-carboxamide (30.6.4). Reacting this with dimethy�lamine gives the desired dacarbazine (30.6.5) 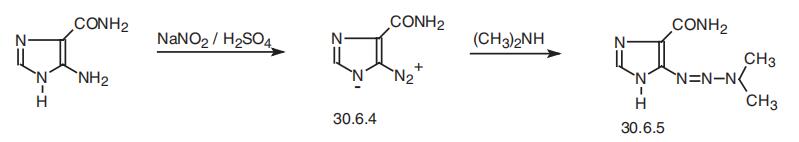
| [Veterinary Drugs and Treatments]
Dacarbazine has been used to treat relapsed canine lymphoma, soft
tissue sarcomas
and melanoma in dogs. In combination with doxorubicin,
dacarbazine has been evaluated to treat dogs with relapsed
lymphosarcoma. Ongoing studies evaluating various protocols are
ongoing for this indication. | [Drug interactions]
Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis. | [Environmental Fate]
The exact mechanism of action of dacarbazine is unknown;
however, several proposed mechanisms have been made
including inhibition of DNA synthesis by acting as a purine
analog, alkylating agent, and interference with sulfhydryl
groups. It is most commonly classified as an alkylating agent in
the triazene group. While the active compound of dacarbazine,
DTIC, is structurally similar to purines, its primary mechanism
of action precludes the agent from being classified as an antimetabolite.
Dacarbazine is a synthetic compound that is
metabolically activated to the active alkylating metabolite
methyl-triazeno-imidazole-carboxamide (MTIC) via the cytochrome
P450 system, primarily CYP1A1, CYP1A2, and CYP
2E1. MTIC is rapidly tautomerized into an inactive derivative,
5-aminoimidazole-4-carboxamide (AIC), which is renally
excreted. The entire process of activating DTIC occurs within
15 min of intravenous infusion. DTIC exerts its actions
throughout all phases of the cellular cycle. The antitumor
effects of this compound are related to the induction of methyl
adducts to DNA. The 70% of alkylation occurs at the N7
position of guanine. The cytotoxic and mutagenic effects of
MTIC are manifested through alkylation of DNA at the O6
guanine position, accounting for 6–8% of methylated bases
formed. This is primarily a result of generation of incorrect base
pairing, leading to DNA double strand breaks and apoptosis. | [Metabolism]
Dacarbazine (DTIC) is assumed to be inactive.
Dacarbazine is extensively metabolised in the liver
by the cytochrome P450 isoenzymes CYP1A2 and
CYP2E1 (and possibly in the tissues by CYP1A1)
to its active metabolite 5-(3-methyl-triazen-1-yl)-
imidazole-4-carboxamide (MTIC), which spontaneously
decomposes to the major metabolite 5-amino-imidazole-
4-carboxamide (AIC). About half of a dose is excreted in
the urine by tubular secretion; 50% as unchanged DTIC
and approximately 50% as AIC. | [storage]
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with dacarbazine you should be trained on its proper handling and storage. Store in a refrigerator or a cool, dry place and protectfrom light. A regulated, marked area should be establishedwhere this chemical is stored in compliance with OSHAStandard 1910.1045. | [Toxicity evaluation]
Dacarbazine is a colorless to an ivory-colored crystalline solid
that must be reconstituted and administered as a parenteral
agent for intravenous injection. It is available as a dry powder in
100, 200, and 500mg vials that when reconstituted have
a standard concentration of 10 mg dacarbazine per 1 ml solution
and a pH of 3–4. Vials of dacarbazine should be refrigerated
(2–8°C) and protected from light. When exposed to light,
dacarbazine is rapidly decomposed to 4-diazoimidazole-5-carboxamide.
When exposed to high temperatures (250–255°C)
dacarbazine decomposes explosively. Dacarbazine is slightly
soluble in water. Dacarbazine may be diluted in either normal
saline or dextrose 5% water. Reconstituted solution is stable for
24 h at room temperature (20°C) and 96 h under refrigeration
(4°C); however, it is recommended by the manufacturer to
use the product within 8 and 72 h, respectively. Dacarbazine
should not be used if it turns pink, as this is a sign of
decomposition. |
| Questions And Answer | Back Directory | [Antineoplastic drug]
Dacarbazine is a new type of purine precursor-class anticancer drug. It can interfere with purine biosynthesis, while having the role of alkylating agents together. It has inhibitory effects on mice sarcoma-180, lung cancer-755, melanoma B16 and leukemia L-1210. It takes effects on the G2 phase of the cell cycle with its main impact on RNA and protein synthesis, followed by DNA. Its oral absorption is not complete with large individual differences. After single-time intravenous injection, it reaches peak within 30 min and disappear after 6h. During the 6 h, the drug excreted from urine account for 30% of the total administered dose. It can’t penetrate through the blood-brain barrier.
Clinical cases mainly apply the citrate salt form of dacarbazine that appears as a white crystalline powder. Its aqueous solution exhibits strong acidicity with the pH being 1.9 to 2.3. It is mainly used for the treatment of malignant melanoma with a better efficacy than hydroxyurea. When being used in combination with vinblastine and BCNU, its efficacy can be improved. It can also be applied to the treatment of squamous cell carcinoma and undifferentiated carcinoma, leiomyosarcoma, fibrosarcoma and so on. However, it is ineffective in treating digestive tract tumors.
| [Pharmacodynamics]
Dacarbazine is the structural analog of the precursor of the purine biosynthesis (see Figure 1), but with its biological effect being similar to the alkylating agent and belongs to the cell cycle non-specific drugs. It has inhibitory effects on several kinds of animal tumors with its major effects on in the G2 phase of the cell cycle with the inhibitory effect being more significant than RNA and protein synthesis.

Figure 1 the structural formula of dacarbazine.
| [Pharmacokinetics]
Because of the incomplete oral absorption and being volatile, dacarbazine can only be subject to the intravenous administration. It first becomes mono-methyl form in the liver through N-demethylation and then metabolized into aminoimidazole carboxamide (AIC) and diazomethane. The active carbon ions are formed from the diazomethane. Dacarbazine has biphasic plasma declining with the half-life being 19 minutes and 5 hours, respectively. It is quickly removed from the tubular secretion. Within 6 hours, about 40% of it is discharged in its prototype form with the primary metabolite in urine being AIC.
This information is edited by Xiongfeng Dai from Chemicalbook | [Clinical application]
It has a better efficacy in treating the spread of malignant melanoma than other anti-cancer drugs. It is most commonly adopted of single-dose treatment and can also be used in combination with BCG, transfer factor and melphalan for immunochemical combination therapy. Applying (ABVD), (MOPP) with alternating method to some poor-outcome patients of advanced Hodgkin's disease can achieve long-term complete remission or even complete curing. It has certain efficacy in the treatment of various kinds of sarcomas, brain tumors, lung squamous cell carcinoma and small cell carcinoma, and children neuroblastoma.
| [Medicine interactions]
1. When being used in combination with other drugs of bone marrow suppression, we should reduce the amount of this product.
2. When being used in combination with interleukin, the risk of allergic reactions will increase.
3. When applying this product to lively vaccines will increase the risk of infection caused by lively vaccines. Patients receiving immunosuppressive should not receive lively vaccines. Leukemia patient in remission period should at least stop three months before being subject to lively vaccines.
4. There is incompatibility when using dacarbazine in combination with hydrocortisone sodium succinate. Therefore, taking together is not suitable.
| [Side effects]
1. Myelosuppression is often the major reason for limiting the dose with mainly affecting the white blood cells and platelets. The effect, compared with traditional mustard, occurs at relatively late time. Neutropenia often occurred in 10 days after treatment while thrombocytopenia occur in 10 to 15 days, but the last two can also be delayed to appear until after 2 to 4 weeks of the last treatment.
2. Nausea and vomiting often occur in 1 to 3 hours after administration with vomiting being sustainable to 12 hours. The side effects can occur in 90% of patients and can be very serious. Application of phenothiazines before medication may be invalid. Under rare circumstances, intractable nausea and vomiting make treatment must be stopped. Most patients can tolerate gradually, and these symptoms can be reduced within 1-2 days after the treatment.
3. It has been reported in patients subjected to dacarbazine of the emergence of a "flu-like" syndrome, including fever, muscle pain and fatigue. Other side effects include: injection site pain, facial flushing, abnormal feeling, hair loss and increased liver enzyme levels. There are also reports of liver necrosis. Dacarbazine is teratogenic and carcinogenic in animals. Allergies are relatively rare.
4. This drug can be put into FDA pregnancy category C.
| [Precautions]
1. it has mutagenic or teratogenic effects. There may be carcinogenic and pregnant women should be disabled.
2. during the administration, the patients should stop breast-feeding.
3. Interference of the diagnostic agent: when using this product, there may be transient increases in the level of blood urea nitrogen, alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase.
4. Patients of varicella zoster should be disabled or should be prohibited for treatment of live virus vaccine.
5. Patients of renal dysfunction, infection should take with caution.
6. During the medication, the patients should be subject to monitoring of regular blood urea nitrogen, creatinine, uric acid, bilirubin, alanine aminotransferase, aspartate aminotransferase and lactate dehydrogenase.
| [Chemical Properties]
It appears as slightly yellow crystalline powder with the M.p. being 205 ℃. It has also been reported of 250-255 ℃ (decomposition). It is easily soluble in acid, slightly soluble in methanol, ethanol but insoluble in water. It can be subject to decomposition when being exposed to heat, light and acid labile.
| [Uses]
It belongs to antineoplastic agent. It is clinically used for the treatment of malignant melanoma with being effective in about 20-30% cases. Combination with vincristine and BCNU can further improve the efficacy. In addition, it also has certain efficacy in treating lung squamous cell carcinoma, sarcoma, leiomyosarcoma and fibrosarcoma but with poor efficacy in treating gastrointestinal cancer.
| [Production method]
Take ethyl cyanoacetate as raw material, go through addition, amination, diazotization, coupling, reduction, formylation, cyclization, and, diazotization, condensation, through intermediate β-imino-β-ethoxy propionate hydrochloride, α-acetamide hydrochloride, α-amidino-α-phenylazo acetamide hydrochloride, 5-amino-imidazole-4-carboxamide hydrochloride to obtain it.
|
|
|