| Identification | More | [Name]
Phenelzine | [CAS]
51-71-8 | [Synonyms]
2-PHENELZINE
(2-PHENYL-ETHYL)-HYDRAZINE
ASINEX-REAG BAS 07650440
BETA-PHENYLETHYLHYDRAZINE
PHENELZINE
PHENETHYL HYDRAZINE
(2-phenylethyl)-hydrazin
1-(2-Phenylethyl)hydrazine
1-hydrazino-2-phenylethane
2-Phenethylhydrazine
Fenelzyna
Fenelzyne
Hydrazine, (2-phenylethyl)-
Hydrazine, phenethyl-
nadil
Nardil
Phenalzine
phenethyl-hydrazin
Phenylethylhydrazine
Stinerval | [EINECS(EC#)]
200-117-9 | [Molecular Formula]
C8H12N2 | [MDL Number]
MFCD00047825 | [Molecular Weight]
136.19 | [MOL File]
51-71-8.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [HazardClass ]
IRRITANT | [Safety Profile]
Poison by ingestion, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: ataxia, somnolence. An experimental teratogen. Experimental reproductive effects. Mutation data reported. Used as an antidepressant. When heated to decomposition it emits toxic fumes of NOx. | [Hazardous Substances Data]
51-71-8(Hazardous Substances Data) | [Toxicity]
LD50 oral in mouse: 130mg/kg |
| Hazard Information | Back Directory | [Description]
Monoamine oxidase inhibitors (MAOIs) were the first antidepressant
drugs introduced during the 1950s. Associated with
many side effects and, in particular, drug–drug and drug–food
interactions, their use declined with the subsequent introduction
of the tricyclic antidepressants and specific serotonin
reuptake inhibitors as first-line treatments for depression. | [Originator]
Nardil ,Parke Davis, US ,1959 | [Uses]
Antidepressant. | [Uses]
MAOIs are used to treat atypical and refractory depression.
They have also been used in the treatment of panic attacks,
narcolepsy, and bulimia. Selective monoamine oxidase B
(MAO-B) inhibitors such as selegiline are used to treat
Parkinson’s disease. | [Uses]
Phenelzine is a MAO inhibitor which is used for treating patients with depressive charac�teristics such as “atypical,” “nonendogenous,” or “neurotic” conditions in which a combi�nation of anxiety, depression, or phobia are observed. Phenelzine is not a drug of first
choice, and it is used in depressions that do not respond to other medicinal drugs. | [Definition]
ChEBI:Phenelzine is a primary amine. | [Manufacturing Process]
To a refluxing solution containing 147.5 grams of 85% hydrazine hydrate in 500 cc of ethanol was added, during a period of 5 hours, 92.5 grams of phenethylbromide (0.50 mol) in 150 cc of ethanol. Stirring and refluxing were continued for two hours. The ethanol was removed by distillation and the residue extracted repeatedly with ether. The ether was dried with potassium carbonate and the product base collected by distillation, BP 74°C/0.1 mm, yield 52.3 grams (77%). The base is reacted with sulfuric acid in propanol to give the sulfate. | [Brand name]
Nardil (Parke-Davis). | [Therapeutic Function]
Psychostimulant | [Mechanism of action]
Phenelzine is a hydrazine MAOI. Its mechanism of action is the prolonged, nonselective,
irreversible inhibition of MAO. Phenelzine has been used with some success in the management of bulimia
nervosa. The MAOIs, however, are potentially dangerous in patients with binge eating and purging behaviors,
and the American Psychiatric Association states that MAOIs should be used with caution in the management
of bulimia nervosa. | [Clinical Use]
MAOI antidepressant | [Synthesis]
Phenelzine, 2-phenylethylhydrazine (7.2.1), is synthesized by reacting
2-phenylethylbromide with hydrazine [42¨C45].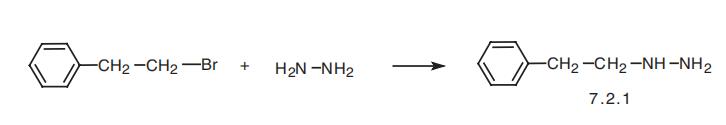
| [Drug interactions]
Potentially hazardous interactions with other drugs
Alcohol: some alcoholic and dealcoholised drinks
contain tyramine which can cause hypertensive crisis.
Alpha-blockers: avoid with indoramin; enhanced
hypotensive effect.
Analgesics: CNS excitation or depression with
pethidine, other opioids and nefopam - avoid;
increased risk of serotonergic effects and convulsions
with tramadol - avoid.
Antidepressants: enhancement of CNS effects and
toxicity. Care with all antidepressants including drug
free periods when changing therapies.
Antiepileptics: antagonism of anticonvulsant effect;
avoid carbamazepine with or within 2 weeks of
MAOIs.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: effects enhanced by clozapine.
Atomoxetine: avoid concomitant use and for 2 weeks
after use.
Bupropion: avoid with or for 2 weeks after MAOIs.
Dapoxetine: risk of hypertensive crisis - avoid.
Diuretics: avoid with indoramin.
Dopaminergics: avoid with entacapone and
tolcapone; hypertensive crisis with levodopa and
rasagiline - avoid for at least 2 weeks after stopping
MAOI; hypotension with selegiline.
5HT1
agonist: risk of CNS toxicity with
sumatriptan, rizatriptan and zolmitriptan - avoid
sumatriptan and rizatriptan for 2 weeks after MAOI.
Methyldopa: avoid concomitant use.
Opicapone: avoid concomitant use.
Sympathomimetics: hypertensive crisis with
sympathomimetics - avoid with methylphenidate.
Tetrabenazine: risk of CNS excitation and
hypertension avoid. | [Environmental Fate]
MAOIs are available orally. Accidental or intentional ingestion
are the most common routes of exposure. | [Metabolism]
Phenelzine is metabolised in the liver by oxidation via
monoamine oxidase, and is excreted in the urine almost
entirely in the form of metabolites. | [Toxicity evaluation]
Monoamine oxidase is the enzyme principally responsible
for degradation of amine neurotransmitters (norepinephrine,
epinephrine, serotonin, and dopamine). There are two
isoenzymes of monoamine oxidase: monoamine oxidase A
(MAO-A) and MAO-B. MAO-A preferentially deaminates
serotonin, norepinephrine, and epinephrine as well as dietary
vasopressors such as tyramine. MAO-B preferentially deaminates
dopamine and phenethylamine. MAOIs block the
monoamine oxidase enzymes leading to neurotransmitter
accumulation. The older MAOIs such as phenelzine, tranylcypromine,
and isocarboxazid were irreversible and nonselective
and inhibited both MAO-A and MAO-B. Moclobemide is
a reversible MAO-A inhibitor used in the treatment of depression.
Selegiline and rasagiline are irreversible selective MAO-B
inhibitors and are approved to treat Parkinson’s disease.
MAOIs do not have any effect on monoamine oxidase
production. Once irreversibly blocked, the monoamine oxidase
enzyme level then regenerates over many weeks. MAOIs may
also stimulate the release of norepinephrine from some nerve
endings while having a sympatholytic effect at postganglionic
terminals. Since selegiline is MAO-B selective, its use does
not result in as many drug–drug and drug–food interactions
as the other MAOIs. |
|
|