| Identification | More | [Name]
Cefotaxime | [CAS]
63527-52-6 | [Synonyms]
Cefotaxima acid
(6R,7R)-3-[(Acetyl-oxy)methyl]-7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)-acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[(acetyloxy)methyl]-7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-, (6R,7R)-
Cefabol
Cephotaxime
(((2-amino-4-thiazolyl)methoxyimino)acetyl)amino)-8-oxo-, (6r-(6alpha,7beta(z) 3-[(Acetyloxy)methyl]-7-[[(2-amino-4-thiazoly)(methoxyimino)acethyl]amino]-8-oxo-5-thia-1-azabicyclo[4,2,0]oct-2-ene-2-carboxylic acid(sodium salt)
Cefotaxime
(6R)-3-(Acetoxymethyl)-7α-[[(2-amino-4-thiazolyl)[(Z)-methoxyimino]acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
(6R,7R)-7α-[2-(2-Amino-4-thiazolyl)-2-[(Z)-methoxyimino]acetylamino]-3-(acetoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Zariviz | [EINECS(EC#)]
264-299-1 | [Molecular Formula]
C16H17N5O7S2 | [MDL Number]
MFCD09028029 | [Molecular Weight]
455.465 | [MOL File]
63527-52-6.mol |
| Hazard Information | Back Directory | [Description]
Like other third-generation
cephalosporins, it has excellent anti-Gram-negative activity and is useful institutionally. It has a
metabolically vulnerable acetoxy group attached to C-3 and loses approximately 90% of its activity when this
is hydrolyzed. This metabolic feature also complicates the pharmacokinetic data, because both active forms
are present and have different properties. Cefotaxime should be protected from heat and light and may color
slightly without significant loss of potency. Like other third-generation cephalosporins, cefotaxime has less
activity against staphylococci but has greater activity against Gram-negative organisms. | [Originator]
Claforan,Hoechst-Roussel,W. Germany,1980 | [Uses]
Cefotaxime has a broad spectrum of antibicrobial use. It acts bactericidally. It is highly
active with respect to Gram-negative microorganisms (E. coli, Citrobacter, Proteus
mirabilis, P. indole, Providencia, Klebsiella, Serratia), and a few strains of Pseudomonas,
H. influenzae that are resistant to other antibiotics. Cefotaxime is less active with respect
to streptococci, pneumococci, meningococci, gonococci, and bacteroides. It is resistant to
the majority of beta-lacatamases of Gram-positive and Gram-negative microorganisms.
This drug is used for severe bacterial infections caused by microorganisms that are sen�sitive to the drug such as peritonitis, sepsis, abdominal infections, infections of the pelvis
minor, infections of the lower respiratory tract, urinary tract, bones, joints, skin, soft tissues,
and infected wounds and burns. Synonyms of this drug are claforan, zarivis, and others. | [Uses]
Cephalosporin antibiotic | [Definition]
ChEBI: Cefotaxime is a cephalosporin compound having acetoxymethyl and [2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino side groups. It has a role as a drug allergen and an antibacterial drug. It is a member of 1,3-thiazoles, an oxime O-ether and a cephalosporin. It is a conjugate acid of a cefotaxime(1-). | [Manufacturing Process]
A solution of 8 g of sodium bicarbonate in about 20 ml of ethanol was
progressively added to 45.55 g of pure 3-acetoxymethyl-7-[2-(2-amino-4-
thiazolyl)-2-methoxyiminoacetamido]-ceph-3-eme-4-carboxylic acid in 100 ml
of distilled water and another 80 ml of ethanol and 4.5 g of activated carbon
were added thereto. The mixture was stirred for 5 minutes and was filtered.
The filter was rinsed with ethanol and the filtrate was evaporated to dryness
under reduced pressure. The residue was taken up in 100 ml of ethanol and
evaporated to dryness again. The residue was dissolved in 100 ml of methanol
and the solution was poured into 2 l of acetone. The mixture was vigorously
stirred and was vacuum filtered. The recovered product was rinsed with
acetone and then ether and dried under reduced pressure to obtain 43.7 g of
a white product which rehydrated in air to obtain a final weight of 45.2 g of
sodium 3-acetoxymethyl-7-[2-(2-amino-4-thiazolyl)-2-
methoxyiminoacetamido]-ceph-3-eme-4-carboxylate. | [Brand name]
Claforan
(Sanofi Aventis). | [Therapeutic Function]
Antibiotic | [Antimicrobial activity]
The aminothiazoyl and methoximino groups at the 7-amino
position confer, respectively, potent activity against many
Gram-negative rods and cocci and stability to
most β-lactamases. Ps. aeruginosa, Sten. maltophilia and other
pseudomonads are often resistant. Brucella melitensis and some
strains of Nocardia asteroides are susceptible. Activity against
L. monocytogenes and B. fragilis is poor. | [Acquired resistance]
Many enterobacteria resistant to other b-lactam agents are
susceptible, but selection of resistant strains with derepressed
chromosomal molecular class C cephalosporinases may occur. Gram-negative bacilli producing variants
of the TEM enzymes (pp. 230–231) are resistant. | [Pharmacokinetics]
Cmax 500 mg intramuscular: 10–15 mg/L after 0.5–1 h
1 g intravenous (15-min infusion): 90 mg/L end infusion
Plasma half-life: c.1 h
Volume of distribution: 32–37 L
Plasma protein binding: c. 40%
Distribution
It is widely distributed, achieving therapeutic concentrations
in sputum, lung tissue, pleural fluid, peritoneal fluid, prostatic
tissue and cortical bone. In patients receiving 2 g every
8 h, mean CSF concentrations in aseptic meningitis were 0.8 mg/L. Levels of 2–15 mg/L can be found in the CSF
in the presence of inflammation after doses of 50 mg/kg by
intravenous infusion over 30 min. A single intraventricular
dose of 40 mg/kg produced levels at 2, 4 and 6 h of 6.4, 5.7
and 4.5 mg/L, respectively.
Metabolism
About 15–25% of a dose is metabolized by hepatic esterases to
the desacetyl form, which may have some clinical importance
because of its concentration in bile and accumulation in renal
failure. Desacetylcefotaxime has about 10% of the activity of
the parent against enterobacteria, less against Staph. aureus.
Its half-life in normal subjects is around 1.5 h.
Excretion
Elimination is predominantly by the renal route, more than
half the dose being recovered in the urine over the first 24 h,
about 25% as the desacetyl derivative. Excretion is depressed
by probenecid and declines in renal failure with accumulation
of the metabolite. In patients with creatinine clearances in the
range 3–10 mL/min, the plasma half-life rose to 2.6 h while
that of the metabolite rose to 10 h. | [Clinical Use]
Cefotaxime is widely used in neutropenic patients, respiratory
infection, meningitis, intra-abdominal sepsis, osteomyelitis,
typhoid fever, urinary tract infection, neonatal sepsis and
gonorrhea. | [Side effects]
Minor hematological and dermatological side effects common
to group 4 cephalosporins have been described.
Superinfection with Ps. aeruginosa in the course of treatment
has occurred. Occasional cases of pseudomembranous colitis
have been reported. | [Synthesis]
Cefotaxime, |á-O-methyloxime acetate (6R, 7R)-7-[2-(2-amino-4-thiazolyl)-
glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic
acid (32.1.2.56), is synthesized by acylating of 7-aminocephalosporanic acid with 2-(2-amino-
4-thiazolyl)-2-methoxyiminoacetic acid, which is protected at the amino group by a trityl pro�tection (32.1.2.54). After removing the trityl protection from the resulting product (32.1.2.55)
with dilute formic acid, the desired cefotaxime (32.1.2.56) is formed. The ethyl ester of 2-(2-
amino-4-thiazolyl)-2-methoxyminoacetic acid necessary for this synthesis, as well as for the
synthesis of a number of other antibiotics of the cephalosporin series, is synthesized from ace�toacetic ester. Nitrosation of acetoacetic ester with nitrous acid gives isonitrosoacetoacetic
ester (32.1.2.49). O-Methylation of the hydroxyl group of obtained product with dimethylsul�fate in the presence of potassium carbonate gives ethyl 2-(methoxyimino)acetoacetate
(32.1.2.50).
Brominating the resulting product with bromine in methylene chloride in the pres�ence of p-toluenesulfonic acid gives 4-bromo-2-methoxyiminoacetoacetic ester (32.1.2.51).
Reacting this with thiourea according to the classic scheme of preparing of thiazoles from |á-
bromocarbonyl compounds and thioamides gives the ethyl ester of 2-(2-amino-4-thiazolyl)-2-
methoxyiminoacetic acid (32.1.2.52). Reacting this with triphenylchloromethane in the
presence of triethylamine results in a trityl protection of the amino group, forming the ethyl
ester of 2-(2-tritylamino-4-thiazolyl)-2-methoxyminoacetic acid (32.1.2.52), which is
hydrolyzed to the acid (32.1.2.54) using sodium hydroxide. The resulting acid (32.1.2.54), as
was already stated, is used for acylating of 7-aminocephalosporanide acid in the presence of
dicyclohexylcarbodiimide, giving tritylated cefotaxime, |á-O-methyloxime acetate 7-[2-(2-
tritylamino)-4-thiazolyl-glycoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo
[4.2.0]oct-2-en-2-carboxylic acid (32.1.2.55). Finally, removing the trityl protection from the
synthesized product (32.1.2.55) using dilute formic acid gives cefotaxime (32.1.2.56).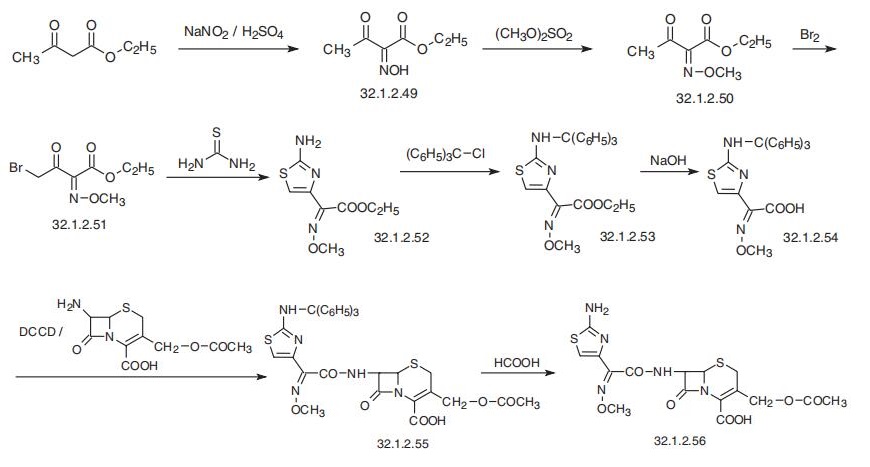
| [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins may be
enhanced. | [Metabolism]
After partial metabolism in the liver to
desacetylcefotaxime and inactive metabolites, elimination
is mainly by the kidneys and about 40-60% of a dose has
been recovered unchanged in the urine within 24 hours;
a further 20% is excreted as the desacetyl metabolite.
Relatively high concentrations of cefotaxime and
desacetylcefotaxime occur in bile and about 20% of a dose
has been recovered in the faeces.
Probenecid competes for renal tubular secretion with
cefotaxime resulting in higher and prolonged plasma
concentrations of cefotaxime and its desacetyl metabolite |
|
|