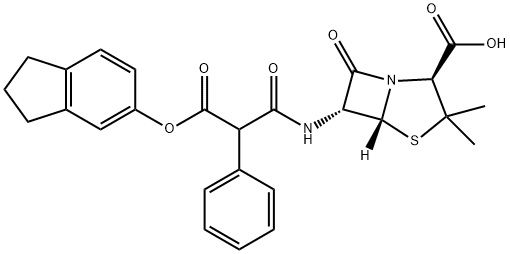
CARINDACILLIN
- CAS No.
- 35531-88-5
- Chemical Name:
- CARINDACILLIN
- Synonyms
- Geopen-U;6α-[[3-(2,3-Dihydro-1H-inden-5-yl)oxy-1,3-dioxo-2-phenylpropyl]amino]penicillanic acid;(2S,5R,6R)-6-[(3-indan-5-yloxy-3-keto-2-phenyl-propanoyl)amino]-7-keto-3,3-dimethyl-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;(2S,5R,6R)-6-[[3-(2,3-dihydro-1H-inden-5-yloxy)-3-oxo-2-phenylpropanoyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;(2S,5R,6R)-6-[[3-(2,3-dihydro-1H-inden-5-yloxy)-3-oxo-2-phenyl-propanoyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;(2S,5R)-6α-[[3-[(2,3-Dihydro-1H-inden-5-yl)oxy]-1,3-dioxo-2-phenylpropyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2β-carboxylic acid;4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[3-[(2,3-dihydro-1H-inden-5-yl)oxy]-1,3-dioxo-2-phenylpropyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)-
- CBNumber:
- CB01091049
- Molecular Formula:
- C26H26N2O6S
- Molecular Weight:
- 494.56
- MDL Number:
- MOL File:
- 35531-88-5.mol
| FDA UNII | 5V278481KE |
|---|
CARINDACILLIN Chemical Properties,Uses,Production
Originator
Geocillin,Roerig,US,1972
Uses
Antibacterial.
Definition
ChEBI: Carindacillin is a penicillin. It is a conjugate acid of a carindacillin(1-).
Manufacturing Process
(A) Preparation of Phenylchlorocarbonyl Ketene: To phenylmalonic acid (20 g)
in ethyl ether (100 ml) there is added phosphorus pentachloride (46 g). A
vigorous reaction occurs. The reaction mixture is refluxed for 4 hours then the
ether partially removed by heating on a steam bath. The reaction mixture
becomes black when about half the ether is removed and the remaining ether
is removed under reduced pressure (at 100 mm). The residue is distilled
under vacuum and the fraction boiling at 75° to 90°C at 1.5 to 4 mm
collected. The product, a yellow liquid, is redistilled at 74°C and 1.5 mm. It
shows a strong peak in the infrared region of the spectrum at 4.69 mu.
Repetition of this procedure but using 10 g of phenylmalonic acid instead of
20 g produces a less vigorous reaction on addition of the phosphorus
pentachloride. The same product is obtained.
(B) Acylation of 6-Aminopenicillanic Acid: To a solution of the aryl
halocarbonyl ketene (0.1 mol) in methylene chloride (sufficient to provide a
clear solution and generally from about 5 to 10 ml per gram of ketene) there
is added the proper alcohol R2OH (0.1 mol), in this case 5-indanyl alcohol.
The reaction mixture is maintained under an atmosphere of nitrogen and
stirred for a period of from 20 minutes to 3 hours, care being taken to exclude moisture. The temperature may range from about -70° to about -
20°C. The infrared spectrum of the mixture is then taken to determine and
confirm the presence of the ketene ester. A solution of 6-aminopenicillanic
acid-triethylamine salt (0.1 mol) in methylene chloride (50 ml) is added and
the mixture stirred at -70° to -20°C for 10 minutes. The cooling bath is then
removed and the reaction mixture stirred continuously and allowed to warm to
room temperature.
Various isolation methods are then spelled out in US Patent 3,679,801.
brand name
Geocillin (Pfizer).
Therapeutic Function
Antibacterial
CARINDACILLIN Preparation Products And Raw materials
Raw materials
Preparation Products
CARINDACILLIN Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12338 | 58 |
| Supplier | Advantage |
|---|---|
| Career Henan Chemica Co | 58 |
| Beijing HuaMeiHuLiBiological Chemical | 58 |