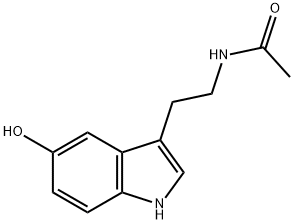
N-ACETYL-5-HYDROXYTRYPTAMINE
- CAS No.
- 1210-83-9
- Chemical Name:
- N-ACETYL-5-HYDROXYTRYPTAMINE
- Synonyms
- Normelatoni;NORMELATONIN;AKOS BC-0717;N-Acetyl-5-HT;Acetylserotonin;N-ACETYLSEROTONIN;ACETYL-SEROTONINE;5-Hydroxymelatonin;O-Demethylmelatonin;MELATONINE EP IMP B
- CBNumber:
- CB2737409
- Molecular Formula:
- C12H14N2O2
- Molecular Weight:
- 218.25
- MDL Number:
- MFCD00005656
- MOL File:
- 1210-83-9.mol
| Melting point | 120-122 °C (lit.) |
|---|---|
| Boiling point | 556.8±40.0 °C(Predicted) |
| Density | 1.268±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | ethanol: 50 mg/mL |
| pka | 10.09±0.40(Predicted) |
| form | powder |
| color | white |
| Stability | Hygroscopic |
| CAS DataBase Reference | 1210-83-9(CAS DataBase Reference) |
| FDA UNII | P4TO3C82WV |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H302-H335-H315 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P-P264-P270-P301+P312-P330-P501 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29339900 | |||||||||
| NFPA 704 |
|
N-ACETYL-5-HYDROXYTRYPTAMINE price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A1824 | N-Acetyl-5-hydroxytryptamine ≥99% (TLC), powder | 1210-83-9 | 100mg | $153 | 2024-03-01 | Buy |
| TCI Chemical | A1277 | N-Acetyl-5-hydroxytryptamine >98.0%(HPLC)(N) | 1210-83-9 | 1g | $453 | 2024-03-01 | Buy |
| TCI Chemical | A1277 | N-Acetyl-5-hydroxytryptamine >98.0%(HPLC)(N) | 1210-83-9 | 100mg | $86 | 2024-03-01 | Buy |
| Cayman Chemical | 14535 | N-Acetylserotonin ≥98% | 1210-83-9 | 50mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 14535 | N-Acetylserotonin ≥98% | 1210-83-9 | 100mg | $68 | 2024-03-01 | Buy |
N-ACETYL-5-HYDROXYTRYPTAMINE Chemical Properties,Uses,Production
Chemical Properties
white to off-white or slightly pink powder
Uses
A metabolite of Melatonin (M215000).
Uses
N-Acetyl-5-hydroxytryptamine has been used to treat neuron cells and to analyze its effects on?Krüppel-like factor 15 (KLF15) expression.
Definition
ChEBI: A member of the class of hydroxyindoles that is the N-acetyl derivative of serotonin.
General Description
N-Acetylserotonin (NAS)/normelatonin acts as a precursor of melatonin in the tryptophan metabolic pathway.
Biological Activity
n-acetylserotonin is an agonist at the melatonin receptors mt1, mt2, and mt3.a melatonin receptor is a g protein-coupled receptor binding melatonin. three types of melatonin receptor have been identified. the mt1 and mt2 receptor subtypes are in humans and other mammals, whereas an additional melatonin receptor subtype mt3 has been identified in amphibia and birds.
Biochem/physiol Actions
N-acetyl-serotonin (NAS/normelatonin) can act as a shelter to neurons due to its protecting ability against oxidative challenges. It can also repress the actions of the transcription factor NF-kappaB. NAS possesses antioxidant and antiaging actions. It has protective action against β-amyloid induced neurotoxicity. It helps to maintain the optimal fluidity of the biological membranes.
in vitro
n-acetylserotonin, a precursor of melatonin, was acetylated from serotonin by arylalkylamine nacetyltransferase (aanat). n-acetylserotonin was found to be able to swiftly activate trkb in a circadian manner. n-acetylserotonin also exhibited antidepressant effect in a trkb-dependent manner. in additioin, n-acetylserotonin could rapidly activate trkb, but not trka or trkc, in a neurotrophin- and mt3 receptor-independent manner. moreover, n-acetylserotonin, but not melatonin, showed a robust antidepressant-like behavioral effect in a trkb-dependent way [1].
in vivo
animal study found that in bdnf knockout mice the administration of n-acetylserotonin could activate trkb. moreover, the endogenous trkb was activated in wild-type c3h/f+/+ mice but not in aanat-mutated c57bl/6j mice, in a circadian rhythm. in addition, trkb activation was found to be high at night in the dark and low during the day [1].
References
[1] jang, s. w.,liu, x.,pradoldej, s., et al. n-acetylserotonin activates trkb receptor in a circadian rhythm. proceedings of the national academy of sciences of the united states of america 107(8), 3876-3881 (2010).
N-ACETYL-5-HYDROXYTRYPTAMINE Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Unipharm pharmaceutical industry Co., Ltd | 536-8266195 +8613953676784 | lee@unipharm-sd.com | China | 1206 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 50000 | 58 |
View Lastest Price from N-ACETYL-5-HYDROXYTRYPTAMINE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2019-07-06 | N-ACETYL-5-HYDROXYTRYPTAMINE
1210-83-9
|
US $100.00 / KG | 1KG | 99% | Cusstomized | Career Henan Chemical Co |
-

- N-ACETYL-5-HYDROXYTRYPTAMINE
1210-83-9
- US $100.00 / KG
- 99%
- Career Henan Chemical Co