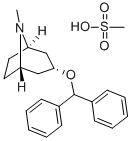
Benztropine mesylate
- CAS No.
- 132-17-2
- Chemical Name:
- Benztropine mesylate
- Synonyms
- mk02;COGENTIN;COGENTINOL;cogentinmesylate;benztropinemesilate;Cogentin,Cogentinol;BENZTROPINE MESYLATE;BENZOTROPINE MESYLATE;Benzatropine mesylate;Benzatropine mesilate
- CBNumber:
- CB2742553
- Molecular Formula:
- C22H29NO4S
- Molecular Weight:
- 403.53
- MDL Number:
- MFCD00074784
- MOL File:
- 132-17-2.mol
| Melting point | 135 °C(lit.) |
|---|---|
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| Water Solubility | Soluble in water (81 mg/ml at 25°C), DMSO (50 mg/ml at 25°C), ethanol (81 mg/ml at 25°C), and ether (very slightly). |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| LogP | 4.962 (est) |
| CAS DataBase Reference | 132-17-2(CAS DataBase Reference) |
| FDA UNII | WMJ8TL7510 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H311+H331 | |||||||||
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352+P312-P304+P340+P311 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 23/24/25 | |||||||||
| Safety Statements | 36/37/39-45 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YM3150000 | |||||||||
| HazardClass | 6.1 | |||||||||
| HS Code | 2939800000 | |||||||||
| NFPA 704 |
|
Benztropine mesylate price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0847 | Benztropine mesylate ≥98% (HPLC) | 132-17-2 | 500mg | $25.26 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1061005 | Benztropine mesylate United States Pharmacopeia (USP) Reference Standard | 132-17-2 | 200mg | $381 | 2024-03-01 | Buy |
| Sigma-Aldrich | 5.09890 | Benztropine - CAS 132-17-2 - Calbiochem An antagonist of muscarinic M1 and M3 receptors that induces robust differentiation of oligodendrocytes precursor cell differentiation (EC?? = 500 nM) and promotes myelination. | 132-17-2 | 50MG | $101 | 2023-01-07 | Buy |
| TCI Chemical | B5592 | Benztropine Mesylate >98.0%(HPLC) | 132-17-2 | 5g | $106 | 2024-03-01 | Buy |
| TCI Chemical | B5592 | Benztropine Mesylate >98.0%(HPLC) | 132-17-2 | 25g | $421 | 2024-03-01 | Buy |
Benztropine mesylate Chemical Properties,Uses,Production
Description
Benztropine mesylate (132-17-2) is a centrally acting M1?muscarinic acetylcholine receptor antagonist (Ki=0.59 nM, rat).1?Also inhibits the dopamine transporter (Ki=160 nM).2?Benztropine mesylate enhances remyelination and significantly decreases clinical severity in the experimental autoimmune encephalomyelitis model of relapsing-remitting multiple sclerosis alone or in combination with immunosuppressive agents.3?Inhibits hepatitis C virus infection.4
Chemical Properties
Benztropine mesylate is a synthetic compound containing structural features found in atropine and diphenhydramine.
Benztropine mesylate is a crystalline white powder, very soluble in water, and has a molecular weight of 403.54. COGENTIN (benztropine mesylate) is supplied as a sterile injection for intravenous and intramuscular use.
Originator
Cogentin,Merck Sharp and Dohme,US,1954
Uses
Benzotropine methanesulfonate is used to treat the symptoms of Parkinson?s disease and is currently in clincial trials for chronic back pain. It shows anti-muscarinic effects comparable to Atropine, and also demonstrates competitive anti-nicotinic action at the nAChR (nicotinic acetylcholine receptor).
Uses
Used as an antiparkinsonian.
Uses
Benztropine mesylate is an anti-histamine and dopamine re-uptake inhibitor. It has been used to study its target identification and mode of action against ebolavirus infection. It has also been used in pharmacological animal studies to evaluate its effect on SLC6A19 (solute carrier family 6 member 19; B0AT1).
Definition
ChEBI: The methanesulfonate salt of benzatropine. An acetylcholine receptor antagonist, it is used in the treatment of Parkinson's disease, and to reduce parkinsonism and akathisia side effects of antipsychotic treatments.
Manufacturing Process
Diphenyldiazomethane was prepared by shaking 7.9 grams of benzophenone
hydrazone and 8.8 grams of yellow mercuric oxide in petroleum ether, filtering
and evaporating off the petroleum ether from the filtrate under reduced
pressure. To the residual diphenyldiazomethane 2.83 grams of tropine and 4.5
ml of benzene were added. The mixture was warmed in a pan of hot water at
about 85°C under reflux for 24 hours after which time the original purple color
had been largely discharged. The reaction mixture was dissolved by adding
benzene and water containing hydrochloric acid in excess of the quantity
theoretically required to form a salt. A rather large amount of water was
required since the tropine benzohydryl ether hydrochloride was not very
soluble and tended to separate as a third phase. The aqueous layer was
separated, washed with benzene and with ether and made alkaline with an
excess of sodium hydroxide. The resulting insoluble oil was extracted with
benzene.
The benzene extracts were dried over potassium carbonate and evaporated
under reduced pressure, leaving a residue of 4.1 grams. The residue (tropine
benzohydryl ether) was dissolved in ether and treated with hydrogen bromide
gas until an acidic reaction was obtained. The precipitate soon became
crystalline and was collected on a filter and dried. The tropine benzohydryl
ether hydrobromide weighed 4.1 grams. Recrystallization from absolute
ethanol gave 3.3 grams of first crop melting at 247°-248°C (dec.).
Twelve grains of tropine benzohydryl ether hydrobromide was converted to the
free base by warming with dilute aqueous sodium hydroxide. The oily base
was extracted with toluene. The toluene extract was washed with water and
then extracted with about 100 ml of water containing 28.1 ml of 1.10 N
methanesulfonic acid, (an equimolecular quantity). The toluene solution was
extracted twice more with fresh portions of water. The combined water
extracts were evaporated under reduced pressure. Residual water was
removed by dissolving the residue in absolute ethanol and evaporating under
reduced pressure several times. Residual alcohol was then removed by
dissolving the residue in acetone and evaporating under reduced pressure
several times. The resulting residue was recrystallized by dissolving in acetone
and adding ether. The crystalline precipitate was collected on a filter, washed with ether and dried at 56°C in vacuo. The tropine benzohydryl ether
methanesulfonate weighed 10.2 grams, MP 138°-140°C.
brand name
Cogentin (Merck).
Therapeutic Function
Antiparkinsonian
General Description
Benztropine mesylate,3α-(diphenylmethoxy)-1αH,5αH-tropane methanesulfonate(Cogentin), has anticholinergic, antihistaminic, and localanesthetic properties. Its anticholinergic effect makes it applicableas an antiparkinsonian agent. It is about as potent ananticholinergic as atropine and shares some of the side effectsof this drug, such as mydriasis and dryness of mouth.Importantly, however, it does not produce central stimulationbut instead exerts the characteristic sedative effect ofthe antihistamines.
Biological Activity
Benztropine mesylate is a centrally acting muscarinic acetylcholine receptor antagonist and dopamine transporter (DAT) inhibitor (IC50 = 118 nM). Benztropine mesylate has been used to treat the symptoms of Parkinson′s disease and is currently in clincial trials for chronic back pain.
Benztropine mesylate serves as an inhibitor of breast cancer stem cells (BCSCs) in vitro and in vivo. It can help in improving the efficacy of chemotherapy in vitro. It is considered as an anti-cancer stem cell (CSC) drug, which can modify tumorigenic properties.
Clinical Use
The tremor and rigidity characteristic of parkinsonismare relieved by benztropine mesylate, and it is particularlyvaluable for those patients who cannot tolerate central excitation(e.g., aged patients). It may also have a useful effectin minimizing drooling, sialorrhea, masklike facies, oculogyriccrises, and muscular cramps.
The usual caution exercised with any anticholinergic inglaucoma and prostatic hypertrophy is observed with thisdrug.
Safety Profile
Poison by ingestion, intravenous, subcutaneous, and intraperitoneal routes. Human systemic effects by ingestion: psychotropic effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also ETHERS.
References
1) Zhang et al. (2001),?Synthesis and biological evaluation of tropane-like 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine (GBR 12909) analogues; J. Med. Chem.,?44?3937 2) Schmitt?et al. (2008),?Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist binding properties; J. Neurochem.,?107?928 3) Deshmukh?et al. (2013),?A regenerative approach to the treatment of multiple sclerosis; Nature,?502?327 4) Mingorance?et al. (2014),?Selective inhibition of hepatitis C virus infection by hydroxyzine and benztropine: Agents Chemother.,?58?3451
Benztropine mesylate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8348 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 28557 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 18958018566; | info@afinechem.com | China | 15377 | 58 |
View Lastest Price from Benztropine mesylate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2022-01-26 | Benztropine mesylate
132-17-2
|
US $0.00 / KG | 1KG | 98.3% | 100 tons | Honest Joy Holdings Limited
|
|
 |
2021-07-27 | BENZTROPINE MESYLATE USP/EP/BP
132-17-2
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2021-07-13 | benztropine mesylate
132-17-2
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Benztropine mesylate
132-17-2
- US $0.00 / KG
- 98.3%
- Honest Joy Holdings Limited
-

- BENZTROPINE MESYLATE USP/EP/BP
132-17-2
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- benztropine mesylate
132-17-2
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
132-17-2(Benztropine mesylate)Related Search:
1of4