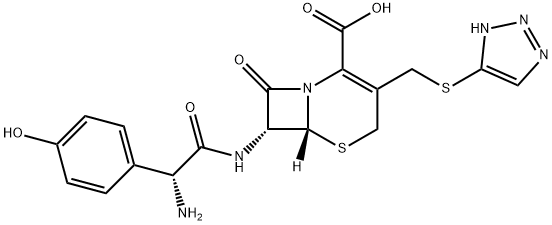
Cefatrizine
- CAS No.
- 51627-14-6
- Chemical Name:
- Cefatrizine
- Synonyms
- s640p;bls640;SKF-60771;eta(r)))-;CEFATRIZINE;cephatriazine;Cephathiamidine;antibioticbl-640;CEPHATHIAMIDINUM;antibioticbl-s640
- CBNumber:
- CB3116636
- Molecular Formula:
- C18H18N6O5S2
- Molecular Weight:
- 462.5
- MDL Number:
- MFCD01940014
- MOL File:
- 51627-14-6.mol
- MSDS File:
- SDS
| Boiling point | 948.1±65.0 °C(Predicted) |
|---|---|
| Density | 1.3806 (rough estimate) |
| refractive index | 1.7000 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO: 95 mg/mL (201.02 mM);Ethanol: 23 mg/mL (48.67 mM) |
| pka | 2?+-.0.50(Predicted) |
| Water Solubility | Water: 95 mg/mL (201.02 mM) |
| CAS DataBase Reference | 51627-14-6(CAS DataBase Reference) |
| FDA UNII | 8P4W949T8K |
| ATC code | J01DB07 |
SAFETY
Risk and Safety Statements
| Toxicity | LD50 in male, female mice, male, female rats (mg/kg): 6880, 6410, 4325, 4325 i.p. (Matsuzaki) |
|---|
Cefatrizine price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Usbiological | 519229 | ETAR | 51627-14-6 | 96Tests | $729 | 2021-12-16 | Buy |
| TRC | C237575 | Cefatrizine | 51627-14-6 | 1g | $1470 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0005842 | CEPHATHIAMIDINUM 95.00% | 51627-14-6 | 10G | $1334.03 | 2021-12-16 | Buy |
Cefatrizine Chemical Properties,Uses,Production
Description
Cefatrizine was synthesized by BristolMyers Co. and Smith Klein & French Laboratories in 1974. It shows two to four times higher activity against gram-positive and four to eight times higher activity against gram-negative bacteria than cephalexin. Cefatrizine also shows excellent oral absorption, and its in vivo activity is 30 to 500 times higher than that of cephalexin.
Originator
Bricef,Bristol Banyu,Japan,1980
Uses
Antidepressant, Selective serotonin reuptake inhibitor
Uses
Cefatrizine is a new orally administered cephalosporin with excellent activity against gram-positive cocci, inhibiting all except enterococci at minimal inhibitory concentrations below 1 μg/ml.
Definition
ChEBI: A cephalosporin compound having (1H-1,2,3-triazol-4-ylsulfanyl)methyl and [(2R)-2-amino-2-(4-hydroxyphenyl)]acetamido side-groups. An antibacterial drug first prepared in the 1970s, it has more recently been found to be n inhibitor of eukaryotic elongation factor-2 kinase (eEF2K), which is known to regulate apoptosis, autophagy and ER stress in many types of human cancers.
Manufacturing Process
A total 6.5 g (1 1.55 mmol) of 7-[D-α-t-butoxycarbonylamino-α-(p-hydroxyphenyl)acetamido]-3-(1,2,3-triazol-5-ylthiomethyl)-3-cephem-4- carboxylic acid was dissolved in 175 ml (98 to 100% formic acid under anhydrous conditions. The mixture was stirred at room temperature for 2.5 hours. Part of the solution, 125 ml, was evaporated under reduced pressure to an amber oil. The oil was then azeotroped 3 times with 70 ml of toluene under reduced pressure. The residue was suspended in an 80:20 H2O-CH3OH solution (700 ml) and stirred for 0.5 hour until most of the solid dissolved, then filtered. The filtration was treated with 1.59 of (Darko) charcoal for about 20 minutes. The charcoal was filtered off through a Celite pad. The solution was then freeze-dried in 9 separate 100 ml round bottom flasks. The freeze-dried material weighed 2.415 g. It was recrystallized in batches of 0.200 g as described above to yield a total of 0.923 g 7-[D-α-amino-α-(p-hydroxyphenyl) acetamidol-3-(1,2,3-triazol-5-ylthiomethyl)-3-cephem-4-carboxylic acid. NMR was consistent, indicating the presence of 0.33 mol of CH3OH.
Therapeutic Function
Antibiotic
Antimicrobial activity
A semisynthetic cephalosporin formulated for oral use. The
spectrum is similar to that of cefalexin but it is more active
against H. influenzae. Wide strain variations in
susceptibility have been reported.
It is only partially absorbed when given by mouth. A
500 mg oral dose achieves a concentration of c. 6 mg/L after
1–2 h. The normal half-life of 2.5 h is extended to 5.5 h
in end-stage renal failure. Distribution resembles that of
cefalexin. It crosses the placenta readily. Plasma protein
binding is 40–60%.
Urinary recovery in 6 h is 35% of an oral dose, producing
urinary levels of 50–1500 mg/L. It is presumed that the
remainder is metabolized, but no metabolites have been
identified.
Apart from some mild diarrhea, it is well tolerated. It is
available in Japan.
Safety Profile
Moderately toxic byintraperitoneal and subcutaneous routes. An experimentalteratogen. Other experimental reproductive effects. Whenheated to decomposition it emits very highly toxic fumesof NOx and SOx.
Cefatrizine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9126 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 28557 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9335 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
View Lastest Price from Cefatrizine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-13 | Cefatrizine
51627-14-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-10 | Cefatrizine
51627-14-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-02 | Cefatrizine
51627-14-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Cefatrizine
51627-14-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Cefatrizine
51627-14-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Cefatrizine
51627-14-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
51627-14-6(Cefatrizine)Related Search:
1of3