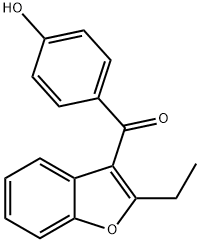
Benzarone
- CAS No.
- 1477-19-6
- Chemical Name:
- Benzarone
- Synonyms
- 2-Ethyl-3-p-hydroxybenzoylbenzofuran;Vasoc;L 2197;Venagil;Benzaron;NSC 82134;Benzarone;Benzarona;Benzaronum;Benzarone CRS
- CBNumber:
- CB6893840
- Molecular Formula:
- C17H14O3
- Molecular Weight:
- 266.29
- MDL Number:
- MFCD00867417
- MOL File:
- 1477-19-6.mol
| Melting point | 124.3° |
|---|---|
| Boiling point | 369.5°C (rough estimate) |
| Density | 1.1601 (rough estimate) |
| refractive index | 1.5490 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 7.68±0.15(Predicted) |
| color | Pale Yellow to Light Brown |
| FDA UNII | 23ZW4BG89C |
| EPA Substance Registry System | Methanone, (2-ethyl-3-benzofuranyl)(4-hydroxyphenyl)- (1477-19-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361 | |||||||||
| Precautionary statements | P501-P202-P201-P280-P308+P313-P405 | |||||||||
| HS Code | 2932.19.1000 | |||||||||
| Toxicity | LD50 ipr-mus: 200 mg/kg AIPTAK 154,94,65 | |||||||||
| NFPA 704 |
|
Benzarone price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | L129305 | Benzarone Aldrich | 1477-19-6 | 10mg | $139 | 2024-03-01 | Buy |
| Sigma-Aldrich | B0490000 | Benzarone European Pharmacopoeia (EP) Reference Standard | 1477-19-6 | b0490000 | $153 | 2024-03-01 | Buy |
| TCI Chemical | E1289 | Benzarone | 1477-19-6 | 1G | $36 | 2024-03-01 | Buy |
| TCI Chemical | E1289 | Benzarone | 1477-19-6 | 5G | $181 | 2024-03-01 | Buy |
| TRC | B185150 | Benzarone | 1477-19-6 | 25mg | $65 | 2021-12-16 | Buy |
Benzarone Chemical Properties,Uses,Production
Chemical Properties
Pale Yellow Solid
Originator
Fragivix,Labaz
Uses
Benzarone is an EYA (Eyes Absents), multifunctional protein involved in organogenesis, inhibitor which makes it a potent anticancer agent. EYA’s are over expressed in ovarian and breast cancers. As well it is involved in the synthesis of Human Uric Acid Transporter 1 inhibitors which may be used to treat kidney diseases.
Definition
ChEBI: Benzarone is a member of 1-benzofurans.
Manufacturing Process
The process of preparation of the 2-ethyl-3-(4'-hydroxybenzoyl)benzofurane includes the next steps:
1. To 1 mol of potassium hydroxide in absolute ethanol is added 1 mole of
salicylic aldehyde. The mixture is brought to boiling point in water bath until
the potassium salt formed is dissolved. One mole of coloroacetone is gradually
added and the solution boiled in a reflux condenser for 2 hours. On cooling
the potassium chloride precipitate is separated off by filtration. The residue is
distilled to give 2-acetyl-1-benzofurane, BP: 135°C/15 mm Hg.
2. It was reduced by hydrazine hydrate in an alkaline medium (by process of
Hyuang-Minlon, J.A.C.S., 1946, 68, 2487) to give 2-ethyl-1-benzofurane BP:
211°-212°C.
3. 2-Ethyl-1-benzofurane is condensed with 2-metoxybenzoyl chloride in the
presence of tin tetrachloride (according to the process described by Bisagni,
J.C.S., 1955, 3694). Thus 2-ethyl-3-(4-methoxybenzoyl)-1-benzofyrane is
obtained. BP: 226°C/15 mm Hg.
4. 1 part of 2-ethyl-3-(4-methoxybenzoyl)-1-benzofyrane is mixed with 2
parts of pyridine hydrochloride and heated at an oil bath at 210°C in N2
current for 1 hour. On cooling 10 parts of 0.5 N HCl are added. A water layer
is mixed with 20 parts of 1% NaOH. The alkaline layer is separated, acidified
with diluted HCl. The dropped precipitate (2-ethyl-3-(4'-hydroxybenzoyl)
benzofurane) is recrystallized from acetic acid. MP: 124.3°C.
brand name
Benzarin;Fragivix (r) forte;Vasco.
Therapeutic Function
Antihemorrhagic
World Health Organization (WHO)
Benzarone is given by mouth and applied topically for treatment of various vascular peripheral disorders.The decision to suspend the marketing authorization results from several reports of toxic hepatitis, including one fatal case from within Germany. The product remains registered in Italy and France.
Biological Activity
Benzarone is an active metabolite of the urate anion transporter 1 (URAT1) inhibitor benzbromarone. It inhibits URAT1 in Xenopus oocytes expressing the human enzyme (IC50 = 2.8 μM). Benzarone also inhibits the tyrosine phosphatase activity of eyes absent homolog 3 (EYA3; IC50 = 17.5 μM), as well as reduces the proliferation and migration of human umbilical vein endothelial cells (HUVECs) when used at a concentration of 7.5 μM. It uncouples oxidative phosphorylation in isolated rat liver mitochondria and induces apoptosis and necrosis in isolated rat hepatocytes. Benzarone (25 μg/g) reduces tumor growth in an A-673 Ewing sarcoma mouse xenograft model.
Safety Profile
Poison by intraperitoneal route. An experimental teratogen. Other experimental reproductive effects. A flammable liquid. When heated to decomposition it emits acrid and irritating smoke and fumes. See also KETONES.
References
[1] W. STüBER H. M. Determination of benzbromarone, bromobenzarone and benzarone in plasma by gas chromatography—mass spectrometry[J]. Journal of Chromatography B: Biomedical Sciences and Applications, 1981, 224 2: Pages 327-331. DOI:10.1016/S0378-4347(00)80171-1.
[2] PRISKA KAUFMANN. Mechanisms of benzarone and benzbromarone-induced hepatic toxicity?[J]. Hepatology, 2005, 41 4: 925-935. DOI:10.1002/hep.20634.
[3] RAM NARESH PANDEY. Structure-activity relationships of benzbromarone metabolites and derivatives as EYA inhibitory anti-angiogenic agents.[J]. PLoS ONE, 2013: e84582. DOI:10.1371/journal.pone.0084582.
[4] MICHAEL F. WEMPE*. Developing Potent Human Uric Acid Transporter 1 (hURAT1) Inhibitors[J]. Journal of Medicinal Chemistry, 2011, 54 8: 2701-2713. DOI:10.1021/jm1015022.
Benzarone Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 | admin@zhanyaobio.com | China | 2136 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21689 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Hebei shuoxi biotechnology co. LTD | +8613081092107 | CHINA | 968 | 58 | |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8348 | 58 |
| Hebei Bonster Technology Co.,Limited | +8613315996897 | bsterltd.wendy@gmail.com | China | 796 | 58 |
| Hebei Runbin Biotechnology Co. LTD | 13180553332 | 2179877681@qq.com | CHINA | 996 | 58 |
View Lastest Price from Benzarone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-02 | Benzbromarone EP Impurity C
1477-19-6
|
US $0.00 / mg | 10mg | 0.98 | 10g | ShenZhen H&D Pharmaceutical Technology Co., LTD | |
 |
2024-01-08 | Benzarone
1477-19-6
|
US $9.00 / KG | 1KG | 99% | 50000tons | Wuhan Boyuan Import & Export Co., LTD | |
 |
2023-08-16 | Benzarone
1477-19-6
|
US $1.00 / KG | 1KG | >99% | 8000T | Weijer International Trade (Hebei) Co., Ltd |
-

- Benzbromarone EP Impurity C
1477-19-6
- US $0.00 / mg
- 0.98
- ShenZhen H&D Pharmaceutical Technology Co., LTD
-

- Benzarone
1477-19-6
- US $9.00 / KG
- 99%
- Wuhan Boyuan Import & Export Co., LTD
-

- Benzarone
1477-19-6
- US $1.00 / KG
- >99%
- Weijer International Trade (Hebei) Co., Ltd