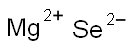magnesium selenide
- CAS No.
- 1313-04-8
- Chemical Name:
- magnesium selenide
- Synonyms
- magnesium selenide;magnesium(III)hydrideselenide
- CBNumber:
- CB6937531
- Molecular Formula:
- MgSe
- Molecular Weight:
- 103.265
- MDL Number:
- MFCD00799782
- MOL File:
- 1313-04-8.mol
| Density | 4.21 |
|---|---|
| solubility | reacts with H2O |
| form | brown cubic crystals |
| color | brown cubic crystals, crystalline |
| Water Solubility | decomposes in H2O [MER06] |
| FDA UNII | N7813M82QS |
| EPA Substance Registry System | Magnesium selenide (MgSe) (1313-04-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS02 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302+H312+H332-H315-H319-H226 |
| Precautionary statements | P501-P261-P270-P240-P210-P233-P243-P241-P242-P271-P264-P280-P370+P378-P337+P313-P305+P351+P338-P362+P364-P303+P361+P353-P332+P313-P301+P312+P330-P304+P340+P312-P403+P235 |
| RIDADR | 3288 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
magnesium selenide Chemical Properties,Uses,Production
Description
Magnesium selenide has the molecular formula of
MgSe and the molecular weight of 103.2652 g/mol. It
is a stable, light gray to brown crystalline powder with
a refractive index, πD=2.44. Its density is 4.21 g/cm3
and it decomposes in water:
MgSe+ 2H2O ? Mg(OH)2+H2Se
Thus, it cannot be prepared by bubbling H2Se gas
through a soluble Mg2+
aqueous solution. MgSe can be
prepared by a variety of other methods. Among the
easiest are the reaction of magnesium oxide and selenium.
Alternately, the two elements can be reacted
together to form the selenide:
MgO(solid)+ Se(solid)+ heat
? MgSe(solid)+ SeO2(gas)
Mg(solid)+ Se(solid)+ heat ? MgSe(solid)
In the first reaction, Se melts at 225°C while the MgO
is stable. Selenium dioxide sublimes at 320°C. The reaction
is carried out in an inert atmosphere at about
550°C. If a tube furnace is used, crystals of SeO2 appear
at the cool end of the tube. In the second reaction, an
inert atmosphere is mandatory and the reaction temperature
is also 550°C (which is below the melting point of
Mg metal).
Magnesium selenide has the CAS number of 1313-
04-8. It is not stable in air and reacts with moisture
to form H2Se, a foul-smelling and toxic gas:
MgSe+H2O ? Mg(OH)2
+H2Se
Chemical Properties
light brown powder(s); unstable in air [MER06]
magnesium selenide Preparation Products And Raw materials
Raw materials
Preparation Products
magnesium selenide Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9020 | 58 |
| Zhengzhou Akem Chemical Co., Ltd | 13303867194 | 3001317055@qq.com | China | 9627 | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 15229059051 | 1027@dideu.com | China | 9944 | 58 |
| Shanghai Yunfu Nano Technology Co., LTD | 15796584478; 15796584478 | 3246531796@qq.com | China | 500 | 58 |
| VI Semiconductor Materials Group Co. Ltd | 0825-7889799 18982573852 | songbai_ding@hotmail.com | China | 208 | 58 |