다우리신
|
|
다우리신 속성
- 녹는점
- 115°
- 알파
- D11 -139° in methanol
- 끓는 점
- 712.3±60.0 °C(Predicted)
- 밀도
- 1.185±0.06 g/cm3(Predicted)
- 저장 조건
- -20°C Freezer, Under inert atmosphere
- 용해도
- DMSO(약간 용해됨), 메탄올(약간 용해됨)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- 9.31±0.45(Predicted)
- 색상
- 페일베이지
- 수용성
- 물에 약간 용해됨
- InChIKey
- AQASRZOCERRGBL-ROJLCIKYSA-N
- SMILES
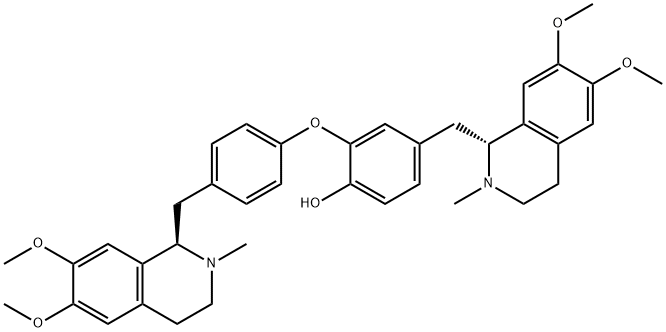
- C1(O)=CC=C(C[C@@H]2C3=C(C=C(OC)C(OC)=C3)CCN2C)C=C1OC1=CC=C(C[C@@H]2C3=C(C=C(OC)C(OC)=C3)CCN2C)C=C1
- CAS 데이터베이스
- 524-17-4
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 그림문자(GHS): |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | |||||||||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||||||||||||||||
| 예방조치문구: |
|
다우리신 C화학적 특성, 용도, 생산
개요
Dauricine is a kind of phenolic aromatic compound which is isolated from Menispermaceae plants such as Asian Menispermum dauricum or Canadian Menispermum dauricum and can be classified as isoquinoline alkaloids.Rhizoma Menispermi is the dry rhizome of Menispermaceae plant Menispermum dauricum. Menispermum dauricum mainly distributes in the northern part of East Asia and is mainly distributed in the north, east and northeast regions of China. The usage of Rhizoma Menispermi as a kind of traditional Chinese medicine was firstly recorded in Kaibao Herbs with its function of detoxifying many drugs, relieving pains, reducing swelling and sore, curing fever and cough, and killing parasites. According to the Chinese Pharmacopoeia, Rhizoma Menispermi in the north of China is different from the herbs used in the south of China known as the same name. Based on the traditional Chinese Medicine theory, Rhizoma Menispermi has the feature of bitterness and coldness, with the meridian tropism in the lung, stomach, and intestine. The main effects of Rhizoma Menispermi are heat-clearing, detoxicating, wind-dispelling and pain-relieving according to its vital function lies in the therapy of swollen sore throat, enteritis diarrhea and rheumatic arthralgia. Alkaloid is the main chemical component of Rhizoma Menispermi, and besides dauricine, there are also other kinds of alkaloids such as dauriciline, O-methyldauricine, and daurisoline in it.
물리적 성질
Appearance: yellowish-white powder or crystal. Solubility: soluble in ethanol, methanol, chloroform, acetone, benzene and slightly soluble in ether. Density: 1.185 g/cm3. Melting point: 115 °C. Boiling point: 712.3 °C at 760 mmHg atmospheric pressure. Specific optical rotation: MeOH-139.역사
Menispermum dauricum has been long used as a traditional Chinese medicine, but the research of its effective components has been slow. Dauricine was firstly separated and structure-confirmed by Fukuda Mayo using alumina column chromatography separation assay in 1964. At the same year, dauricine was first synthesized by two Japanese scientists, Tetsuji Tamayama and Rtani Rikazu, by means of Arndt-Eistert reaction and Bischler-Napieralski reaction. Thereafter, lots of scientists from various countries successively separated daurinoline, Menispermum Xinlin alkali, Menispermum succinylcholine alkali, chelilanthifoline, stepholidine, and many other monomeric components of Menispermum dauricum.The study of dauricine in China started from the Cultural Revolution Period. Under the guidance of integrated traditional Chinese and Western medicine, a large number of effective components in traditional Chinese herbs were extracted, separated and applied to clinic. Dauricine was firstly reported on the mainland as an active ingredient in herbs which can clear away heat and toxic materials. Moreover, dauricine was also used as an antineoplastic and muscle relaxant. There were many medicines whose principal component was dauricine had been under clinical research for its role of cardiovascular protection since the 1980s, most of these medicines were stagnating in phase II clinical trials.
용도
Dauricine is a bisbenzylisoquinoline allkaloid derivative that displays a noted number of pharmaceutical properties. It is known to induces severe lung toxicity in animals.Indications
Drugs whose main component is dauricine are still in clinical trials. Several formulations of dauricine have currently been developed, including tablets, capsules, injections, and so on. The main indications of dauricine in clinical trials are antiplatelet,antihypertensive and anti-arrhythmic.정의
ChEBI: A bisbenzylisoquinoline alkaloid resulting from the formal oxidative dimerisation of 4-{[(1R)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl]methyl}phenol by attachment of the phenolic oxygen of one molecule to the benzene ring f the second (ortho to the phenolic hydroxy group of the latter).Pharmacology
The main pharmacological actions of dauricine are as follows; Anti-arrhythmia: Dauricine can slow the heart rate and prolong conduction time in sinus rhythm, therefore reducing the incidence of arrhythmia.Anti-myocardial ischemia: Dauricine has protective effect on myocardial ischemia and can improve hemodynamic disturbances during ischemia which is supposed to produce effect via mechanism of improving myocardial metabolism and anti-oxidation.
Anti-cerebral ischemia: Dauricine has an effective regulation on gene expression of Bcl-2 and Bax in cortical neurons after the ischemic brain injury, protecting against cerebral ischemia.
Anti-cerebral ischemia/reperfusion injury: Dauricine can inhibit apoptosis induced by cerebral ischemia/reperfusion injury by upregulating Bcl-2 expression and downregulating Bax expression. Also dauricine has the effect of anti-oxidation.
Antitumor activity: Dauricine can affect TGF-beta signaling pathway via upregulating gene expression of DPC4, and then the gene expression of P53 and P16 in tumor tissue, and finally reduce the gene expression of hFGF in tumor tissue. Thereby, the growth of various tumor cells could be inhibited.
The investigations of metabolism in vivo of dauricine indicate that it is rapidly and widely distributed in body and the drug concentration in organs is significantly higher than that in plasma, and this is possibly related to its strong lipid solubility and easy access to cells.
Clinical Use
No medicine in the market takes dauricine as the main component up till now. Many animal experiments and clinical studies suggest that dauricine can reverse the effect of MDR as verapamil. Since the clinical application of the classical drug verapamil is limited for the serious toxic side effects, the moderate pharmacological effect of dauricine has a vital clinical significance. The main side effect of dauricine by oral absorption is gastrointestinal reaction, such as diarrhea, nausea, abdominal distension, and so on. In addition, dauricine can accumulate in the body, leading to hepatorenal toxicity and central nervous system toxicity.다우리신 준비 용품 및 원자재
원자재
준비 용품
다우리신 공급 업체
글로벌( 210)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12468 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 3001 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 |
sales@biopurify.com | China | 3424 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8829 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| BOC Sciences | +1-631-485-4226 |
inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 |
ft-sales@nature-standard.com | CHINA | 1923 | 58 |
다우리신 관련 검색:
페루라산 다우리신
13,14-Didehydromatridin-15-one
Chelerythrine
Silibinin
D-Tetrandrine
Swertiamarine
3-(1-(Dimethylamino)ethyl]phenol
3-PHENOXYPHENETHYLAMINE
SALSOLIDINE
MAGNOLINE
(R)-1-(3,4-Dimethoxyphenyl)ethylamine
(S)-1-(3,4-Dimethoxyphenyl)ethylamine
1,2-Benzenediol, 4-(1-aminoethyl)-, (R)- (9CI)
(R)-3-(1-AMINOETHYL)PHENOL
GS 389
Phenol, 4-(1-aminoethyl)-2-methoxy-, (R)- (9CI)
Phenol, 5-(1-aminoethyl)-2-methoxy-, (R)-