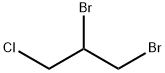
1,2-디브로모-3-클로로프로판
|
|
1,2-디브로모-3-클로로프로판 속성
- 녹는점
- 6°C
- 끓는 점
- 195°C
- 밀도
- 2.05
- 증기압
- 0.8 at 21 °C (quoted, Verschueren, 1983)
- 굴절률
- n/D 1.5542
- 인화점
- 77 °C
- 저장 조건
- Refrigerator
- 용해도
- 오일, 디클로로프로판 및 이소프로판올과 혼합 가능(Windholz et al., 1983)
- 물리적 상태
- 밀도가 높은 액체
- 색상
- 무색~연황색
- 수용성
- 0.123g/100mL
- Merck
- 14,3020
- BRN
- 1732077
- Henry's Law Constant
- 2.49 x 10-4 atm?m3/mol at 20 °C (approximate - calculated from water solubility and vapor pressure)
- 노출 한도
- OSHA PEL: TWA 1 ppb.
- 안정성
- 안정적인. 가연성. 강한 산화제, 화학적 활성 금속 및 그 합금과 호환되지 않습니다. 일부 고무 기반 재료를 공격할 수 있습니다.
- CAS 데이터베이스
- 96-12-8(CAS DataBase Reference)
- IARC
- 2B (Vol. 20, Sup 7, 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-46-60-25-48/20/22-52/53-39/23/24/25-23/24/25-11 | ||
| 안전지침서 | 53-45-61-36/37-16-7 | ||
| 유엔번호(UN No.) | 2872 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TX8750000 | ||
| 위험 참고 사항 | Toxic | ||
| TSCA | T | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | III | ||
| HS 번호 | 2903793090 | ||
| 유해 물질 데이터 | 96-12-8(Hazardous Substances Data) | ||
| 독성 | LD50 in rats, mice (g/kg): 0.17, 0.26 orally (Torkelson) | ||
| 기존화학 물질 | KE-09931 | ||
| 유해화학물질 필터링 | 97-1-419;06-4-56 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 1,2-디브로모-3-클로로프로판 및 이를 0.1% 이상 함유한 혼합물 |
1,2-디브로모-3-클로로프로판 C화학적 특성, 용도, 생산
화학적 성질
colourless to slightly yellow liquid물리적 성질
Colorless when pure, however, technical grades are yellowish to dark brown. Pungent odor at high concentrations용도
Soil fumigant; nematocide; intermediate in organic synthesis.생산 방법
DBCP is produced by liquid phase addition of bromine to allyl chloride. It was first produced commercially in the United States in 1955.일반 설명
A colorless liquid. Denser than water. Flash point 170°F. Boiling point 195°F. Toxic by ingestion and inhalation. Used as a pesticide and fumigant.공기와 물의 반응
Flammable. Soluble in water. Hydrolyzed in alkali.반응 프로필
1,2-Dibromo-3-chloropropane reacts with chemically active metals such as aluminum, magnesium, tin and their alloys. 1,2-Dibromo-3-chloropropane will attack some rubber materials and coatings.건강위험
Inhalation of vapors or dust is extremely irritating. May cause burning of eyes and flow of tears. May cause coughing, difficult breathing and nausea. Brief exposure effects last only a few minutes. Exposure in an enclosed area may be very harmful. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.화재위험
Some of these materials may burn, but none ignite readily. Containers may explode when heated.농업용
Nematicide, Fumigant: DBCP has been used in agriculture as a nematicide since 1955, being supplied for such use in the forms of liquid concentrate, emulsifiable concentrate, powder, granules, and solid material. A rebuttable presumption against registration for pesticide uses was issued by U.S. EPA on September 22, 1977, on the basis of oncogenicity and reproductive effects. Then, as of November 3, 1977, EPA in a further action suspended all registrations of end-use products, subject to various specific restrictions. Not listed as registered in EU countries.상품명
BBC 12®; FUMAGONE®; FUMAZONE®[C]; MEMATOCIDE®; NEMABROM®; NEMAFUM®; NEMAGON®[C]; NEMAGON SOIL FUMIGANT®[C]; NEMANAX®; NEMAPAZ®; NEMASET®; NEMATOCIDE®[C]; NEMATOX®; NEMAZON®; OS 1897®; OXY BCP®[C]; SD 1897®Safety Profile
Confirmed human carcinogen with experimental carcinogenic and teratogenic data. Poison by ingestion, inhalation, and subcutaneous routes. Moderately toxic by skin contact. An eye and severe skin irritant. Narcotic in high concentrations. Has been implicated in causing human sterihty in male factory workers. Human mutation data reported. A soil fumigant. Combustible. When heated to decomposition it emits toxic fumes of Cl and Br-. See also CHLORIDES and BROLVIDES.Carcinogenicity
1,2-Dibromo-3-chloropropane is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity fromstudies in experimental animals.환경귀착
Biological. Biodegradation is not expected to be significant in removing 1,2-dibromo- 3-chloropropane. In aerobic soil columns, no degradation was observed after 25 days (Wilson et al., 1981a).Soil. Soil water cultures converted 1,2-dibromo-3-chloropropane to n-propanol, bromide and chloride ions. Precursors to the alcohol formation include allyl chloride and allyl alcohol (Castro and Belser, 1968). The reported half-life in soil is 6 months (Jury et al., 1987).
Groundwater. According to the U.S. EPA (1986) 1,2-dibromo-3-chloropropane has a high potential to leach to groundwater.
Chemical/Physical. 1,2-Dibromo-3-chloropropane is subject to both neutral and basemediate hydrolysis (Kollig, 1993). Under neutral conditions, the chlorine or bromine atoms may be displaced by hydroxyl ions. If nucleophilic attack occurs at the carbon-chlorine bond, 2,3-dibromopropanol is formed which reacts further to give 2,3-dihydroxybromopropane via the intermediate epibromohydrin. 2,3-Dihydroxybromopropane will undergo hydrolysis via the intermediate 1-hydroxy-2,3-propylene oxide which further reacts with water to give glycerol. If the nucleophilic attack occurs at the carbon-bromine bond, 2- bromo-3-chloropropanol is formed which further reacts forming the end product glycerol (Kollig, 1993). If hydrolysis of 1,2-dibromo-2-chloropropane occurs under basic conditions, the compound will undergo dehydrohalogenation to form 2-bromo-3-chloropropene and 2,3-dibromo-1-propene as intermediates. Both compounds are subject to further attack forming 2-bromo-3-hydroxypropene as the end product (Burlinson et al., 1982; Kollig, 1993). The hydrolysis half-life at pH 7 and 25°C was calculated to be 38 years (Burlinson et al., 1982; Ellington et al., 1986).
Emits toxic chloride and bromide fumes when heated to decomposition (Lewis, 1990).
1,2-디브로모-3-클로로프로판 준비 용품 및 원자재
원자재
Liquid bromine
염화알릴
Propane, 2-bromo-1-chloro-3-fluoro-
1-브로모-3-클로로-2-플루오로프로판
HCFC-262ea
Catechylphosphorus Tribromide
에피클로로하이드린
준비 용품
1,2-디브로모-3-클로로프로판 공급 업체
글로벌( 142)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 |
info@konochemical.com | China | 2995 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34571 | 58 |
| Alfa Chemistry | +1-5166625404 |
Info@alfa-chemistry.com | United States | 21317 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 9020 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 43348 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57511 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131980 | 58 |
1,2-디브로모-3-클로로프로판 관련 검색:
1,3-디브로모프로판 에피클로로하이드린 1-브로모-3-클로로프로페인 1-클로로프로판 클로로디플루오르메탄 클로로아세트산 디메틸-1,2-디브로모-2,2-디클로로에틸포스페이트 1,2-디브로모-3-클로로프로판
1,1,1,2,3,3,3-HEPTACHLOROPROPANE
1,2-Dibromopropane
2,3-Dibromopropionyl chloride
1,2,3,4,5-Pentabromo-6-chlorocyclohexane
1,2-DIBROMO-2,3-DICHLOROPROPANE
1,2-DIBROMO-1-CHLOROPROPANE
INDOLIZINE
1,2-DIBROMOETHANE & 1,2-DIBROMO-3-CHLOROPROPANE
1,3-DIBROMO-2-CHLOROPROPANE
1.2-DIBROMO-3-CHLOROPROPANE SOLUTION 100UG/ML IN METHANOL 1ML