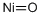산화 니켈 C화학적 특성, 용도, 생산
화학적 성질
Nickel(II) oxide is Brownish black or black powder.
용도
Nickel(II) oxide is used in the preparation of nickel alloys including nickel steel alloys, manufacture of glass and porcelain paints. Further nickel oxide is the main component in nickel-iron battery (Edison battery), nickel-cadmium rechargeable battery, and also used in ceramic industry for making frits and porcelain glazes. It also acts as a hydrogenation catalyst. NiO/CNTs (nickel oxide/carbon nanotubes) could be a potential cathode catalyst for oxygen reduction reaction (ORR) in microbial fuel cells (MFCs). It is blended with other high purity oxides and used in a variety of semiconductor applications such as thermistors and varistors.
제조 방법
Nickel oxide is prepared by heating pure nickel powder with oxygen at a temperature above 400°C. In some commercial processes, green nickel oxide is made by heating a mixture of nickel powder and water in air at 1,000°C. Adding some nickel oxide to the above mixture enhances the rate of reaction. An alternative method of preparation of the green oxide involves thermal decomposition of an oxo acid salt of nickel at elevated temperatures. Thus, nickel nitrate, nickel sulfate or, more conveniently, nickel carbonate when heated at 1,000°C, yields the green oxide. The black oxide, on the other hand, is produced at a lower temperature from incomplete calcination of the carbonate or nitrate salt at 600°C. The oxygen content of the black form is slight-ly greater than its green counterpart.
화학 반응
Nickel(II) oxide is insoluble in water but soluble in acids as long as it has not been
ignited at a high temperature (under these latter conditions it is converted into grey-black
octahedra having a metallic lustre). It reacts reversibly with hydrogen, the reaction
NiO+H2 ? Ni+H20
proceeding from left to right at relatively low temperatures in a stream of hydrogen.
일반 설명
Nickel(II) oxide (NiO) is a metal oxide based nanomaterial with a good semiconducting property. Nanosized nickel oxide can be found in a variety of morphologies which include nanoflowers, spheres, wires, and tubes. It exhibits high performance in applications which require charge transfer and charge transport based processes. It can be prepared by a variety of physical and thermal methods such as sol-gel, hydrothermal and solvothermal techniques.
위험도
Confirmed carcinogen.
Safety Profile
Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Poison by intratracheal,
intravenous, and subcutaneous routes.
Mutation data reported. Can react violently
with fluorine, hydrogen peroxide, hydrogen
sulfide, iodine, barium oxide + air. See also
NICKEL COMPOUNDS.
산화 니켈 준비 용품 및 원자재
원자재
준비 용품