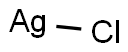염화은 C화학적 특성, 용도, 생산
화학적 성질
Silver chloride, AgCl, is a white,granular powder that darkens on exposure to light,finally turning black.It exists in several modifications differing in behavior toward light and solubility in various solvents. Soluble in ammonium hydroxide, concentrated sulfuric acid, and sodium thiosulfate and potassium bromide solutions, very slightly soluble in water, can be melted, cast, and fabricated like a metal. Derived by heating a silver nitrate solution and adding hydrochloric acid or salt solution. The whole is boiled, then filtered. This must take place in the dark or under a ruby-red light. Used in photography,photometry and optics, batteries, photochromic glass,silver plating,production of pure silver, and as an antiseptic. Single crystals are used for infrared absorption cells and lens elements and as a lab reagent
용도
Employed in Silver plating. Owing to its characteristic of reversible reduction to silver metal, it is used in photochromic lenses. Used as a cathode in sea water activated batteries. In electrochemistry, silver chloride electrode is used for potentiometric measurements. It serves as an antidote for mercury poisoning, and eliminates mercury from body. It is used in glass manufacturing industry. It is useful in the production of bandages, wound healing products and inglaze lustre, personal deodorant products, as well as for long term preservation of drinking water in water tanks; its pharmaceutical composition finds use as an antibacterial agent.
주요 응용
?AgCl is very important as a linear polarizer in the infrared region (λ: 2–23 mm). The refractive index is almost constant in the infrared region and the polarization angle is almost independent of wavelength. The polarization angles are 63°43' (3 mm), 63°20' (10 mm), and 63°33' (20 mm), showing the difference of angle below 18 for λ: 2–23 mm. The polariscope is fabricated typically by arranging the six sheets of plates with the thickness of 0.5 mm in the shape of roof type. Bakelite or plastic is good for the material of the holder case.
정의
T3DB: Silver chloride is a chloride of silver that occurs naturally as the mineral chlorargyrite. It is used to make photographic paper and pottery glazes. It is also found in stained glass colorants, bandages, and other wound healing products, and may be used as an antidote to mercury poisoning. Silver is a metallic element with the chemical symbol Ag and atomic number 47. It occurs naturally in its pure, free form, as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite.
위험도
As for silver.
Purification Methods
Recrystallise it from conc NH3 solution by acidifying with HCl, filtering off the solid, washing it with H2O and drying it in a vacuum. It is soluble in NH3 and should be kept in the dark.
염화은 준비 용품 및 원자재
원자재
준비 용품