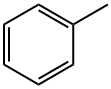
톨루엔
|
|
톨루엔 속성
- 녹는점
- -93 °C (lit.)
- 끓는 점
- 110-111 °C (lit.)
- 밀도
- 0.865 g/mL at 25 °C (lit.)
- 증기 밀도
- 3.2 (vs air)
- 증기압
- 22 mm Hg ( 20 °C)
- 굴절률
- n
/D 1.496(lit.)
- 인화점
- 40 °F
- 저장 조건
- 0-6°C
- 산도 계수 (pKa)
- 40(at 25℃)
- 물리적 상태
- 액체
- 색상
- 무색의
- Specific Gravity
- 0.865~0.870(20/20℃)(Ph.Eur.)
- 냄새
- 0.16~37ppm(평균 = 1.6ppm)에서 감지 가능한 벤젠과 같은 방향족 냄새
- 상대극성
- 0.099
- 폭발한계
- 7%
- Odor Threshold
- 0.33ppm
- 수용성
- 0.5g/L(20℃)
- Merck
- 14,9529
- BRN
- 635760
- Henry's Law Constant
- 1.05 at 40 °C, 1.68 at 50 °C, 2.62 at 60 °C, 3.15 at 70 °C, 3.97 at 80 °C (headspace-GC, Vane et al., 2001)
- 노출 한도
- TLV-TWA 100 ppm (~375 mg/m3) (ACGIH, NIOSH, and MSHA), 200 ppm (~750 mg/ m3) OSHA; ceiling 300 ppm, peak 500 ppm/ 15 min (OSHA); STEL 150 ppm (ACGIH).
- Dielectric constant
- 2.4(20℃)
- LogP
- 2.73 at 20℃
- CAS 데이터베이스
- 108-88-3(CAS DataBase Reference)
- IARC
- 3 (Vol. 47, 71) 1999
- NIST
- Toluene(108-88-3)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xn,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-38-48/20-63-65-67-39/23/24/25-23/24/25 | ||
| 안전지침서 | 36/37-46-62-45-16-7 | ||
| 유엔번호(UN No.) | UN 1294 3/PG 2 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | XS5250000 | ||
| F 고인화성물질 | 3-10 | ||
| 자연 발화 온도 | 480 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29023000 | ||
| 유해 물질 데이터 | 108-88-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 7.53 g/kg (Smyth) | ||
| IDLA | 500 ppm | ||
| 기존화학 물질 | KE-33936 | ||
| 유해화학물질 필터링 | 97-1-298 | ||
| 사고대비 물질 필터링 | 28 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 톨루엔 및 이를 85% 이상 함유한 혼합물 |
톨루엔 C화학적 특성, 용도, 생산
개요
매트포르민은 간에서의 포도당 생산을 억제함으로써 고혈당 상태에서 혈당을 낮춘다.일반적으로 2형 당뇨병 환자는 정상적인 사람보다 3배 정도 많이 포도당을 생산하는데, 매트포르민 요법은 과다한 포도당 생산을 3분의 1정도로 낮춰 정상 수준이 되게 한다.개요
피루브산은 생화학에서 중요한 화합물이다. 피루브산은 해당과정으로 알려진 포도당의 대사 산물이다. 1분자의 포도당은 2분자의 피루브산으로 분해되며, 다음의 두 가지 방법 중 하나를 사용하여 추가적인 에너지를 공급한다. 피루브산은 시트르산 회로(또는 트라이카복실산(TCA) 회로 또는 크렙스 회로)로 알려진 일련의 반응들에 대한 주된 시작 기질인 아세틸-CoA로 전환된다. 또한 피루브산은 보충대사 반응(anaplerotic reaction)에 의해 옥살아세트산으로 전환되며, 이는 시트르산 회로의 중간생성물을 보충한다.개요
Toluene is a clear, colourless liquid with a sweet, benzene-like odour. Toluene occurs naturally in crude oil and in the toluene tree. It is also produced in the process of making gasoline and other fuels from crude oil and making coke from coal. Toluene is used in making paints, paint thinners, fingernail polish, lacquers, adhesives, and rubber and in some printing and leather tanning processes. Toluene is also used in the production of polymers used to make nylon, plastic soda bottles, and polyurethanes and for pharmaceuticals, dyes, cosmetic nail products, and the synthesis of organic chemicals.Toluene has been reported as the most commonly abused hydrocarbon solvent, primarily through ‘glue sniffing’. The common possibilities of exposure to high levels of toluene include indoor air from the use of household products such as paints, paint thinners, adhesives, synthetic fragrances, and many other sources.
화학적 성질
Toluene is a clear, colorless, flammable liquid with a sweet/pungent odor. It is extensively used as a solvent in different industries, i.e., rubber chemical manufacturing, drugs and pharmaceuticals, thinner for inks, paints dyes, and perfume manufacturing. It is a natural constituent of crude oil and is produced from petroleum refi ning and coke-oven operations. Toluene occurs naturally as a component of crude oil and occurs in petroleum refi ning and coke oven operations. Occupational workers associated with several kinds of activities, such as manufacturing of dyes, printing inks, painting automobile mechanics, gasoline manufacturers, shippers, and retailers, adhesives and coatings manufacturers and applicators, audio-equipment product workers, chemical industry workers, coke-oven workers, fabric manufacturers (fabric coating), sites of hazardous wastes, linoleum manufacturers, in pharmaceutical manufacturing, printing works, shoe manufacturing industry, become exposed to toluene.물리적 성질
Colorless, clear, flammable liquid with a pleasant, sweet or paint-like odor similar to benzene. At 40 °C, the lowest concentration at which an odor was detected were 960 μg/L. Similarly at 25 °C, the lowest concentration at which a taste was detected was 960 μg/L (Young et al., 1996). Experimentally determined detection and recognition odor threshold concentrations were 600 μg/m3 (160 ppbv) and 7.0 mg/m3 (1.9 ppmv), respectively (Hellman and Small, 1974). Leonardos et al. (1969) reported higher odor threshold concentrations for toluene derived from coke (4.68 ppmv) and petroleum (2.14 ppmv). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 24 and 140 μg/L, respectively (Alexander et al., 1982). An odor threshold concentration of 330 ppbv was determined by a triangular odor bag method (Nagata and Takeuchi, 1990). Cometto-Mu?iz and Cain (1994) reported an average nasal pungency threshold concentration of 29,574 ppmv.역사
Toluene is a clear, flammable, aromatic hydrocarbon liquid with a smell similar to benzene. It is also called methylbenzene, indicating that a methyl group has been added to one of benzene’s carbon atoms. Toluene was first isolated by Pierre-Joseph Pelletier (1788–1842) and Philippe Walter (1810–1847) in 1837. The name toluene comes from the South American tree Toluifera balsamum. Henri-Etienne Sainte-Claire Deville (1818–1881) isolated toluene from the tree’s gum, Tolu balsam, in 1841.The main source of toluene is from the catalytic reforming of naphthas during petroleum processing. During this process cycloalkanes are dehydrated, forming aromatics such as toluene and xylene along with hydrogen. Toluene can also be obtained from the pyrolysis of gasoline. It is a by-product when styrene is produced and can also be produced from coal tar, which was its main source in the first half of the 20th century.
용도
Toluene is derived from coal tar as well aspetroleum. It occurs in gasoline and manypetroleum solvents. Toluene is used to producetrinitrotoluene (TNT), toluene diisocyanate,and benzene; as an ingredient fordyes, drugs, and detergents; and as an industrialsolvent for rubbers, paints, coatings, andoils.생산 방법
Benzene is produced from toluene through a process called hydrodealkylation. In thisprocess, toluene reacts with hydrogen in the presence of a chromium, platinum, or molybdenumcatalysts at temperatures of several hundred degrees Celsius and pressures of about50 atmospheres: C6H5CH3 + H2 → C6H6 + CH4. Toluene can also be used to producephenol, (C6H5OH), benzoic acid (C6H5COOH), and benzaldehyde (C6H5CHO). Nitratedforms of toluene produce explosive compounds; the most common of these is TNT (SeeTrinitrotoluene).제조 방법
Toluene is the starting material for the production of tolylene diisocyanate (TDI),the process may be varied to give products of differing isomer contents. The nitration of toluene (with a nitrating mixture containing 20% nitric acid, 60% sulphuric acid and 20% water at 30-45°C) gives a mixture of 2-nitrotoluene (about 60%) and 4- nitrotoluene (40%). If this mixture is nitrated further (with a mixture of 35% nitric acid and 65% sulphuric acid at 65-80°C) without separation, the product is a mixture of2,4-dinitrotoluene (about 80%) and 2,6-dinitrotoluene (20%). If, on the other hand, the mixed mononitrates are separated (by distillation), then further nitration of the 2-nitrotoluene yields a mixture of 2,4-dinitrotoluene (about 65%) and 2,6-dinitrotoluene (35%) whilst further nitration of the 4-nitrotoluene gives only 2,4-dinitrotoluene.정의
ChEBI: The simplest member of the class toluenes consisting of a benzene core which bears a single methyl substituent.일반 설명
A clear colorless liquid with a characteristic aromatic odor. Flash point 40°F. Less dense than water (7.2 lb / gal) and insoluble in water. Hence floats on water. Vapors heavier than air. May be toxic by inhalation, ingestion or skin contact. Used in aviation and automotive fuels, as a solvent, and to make other chemicals.공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
Toluene reacts vigorously with allyl chloride or other alkyl halides even at minus 70° C in the presence of ethyl aluminum dichloride or ethyl aluminum sesquichloride. Explosions have been reported [NFPA 491M 1991]. Incompatible with strong oxidizing agents. When added to a tank of sulfur dichloride, the tank over pressurized and ruptured in a reaction thought to be catalyzed by iron or iron(III) chloride [Chem. Eng. News, 1988, 66(32), 2].위험도
Flammable, dangerous fire risk. Explosive limits in air 1.27–7%. Toxic by ingestion, inhalation, and skin absorption. Visual impairment, female reproductive effects, and pregnancy loss. Questionable carcinogen.건강위험
Exposures to toluene cause adverse health effects to animals and humans. The symptoms of toxicity and poisoning include, but are not limited to, mild irritation to the skin, headache, nausea, and effects on the CNS. Prolonged exposure to high concentrations of toluene causes disturbances in vision, dizziness, nausea, CNS depression, paresthesia, and sudden collapse. The acute oral LD50 value of toluene in laboratory rats has been reported as 636–7300 mg/kg. Exposure to toluene has been reported to cause rapid and severe corneal damage and conjunctiva infl ammation. The acute dermal LD50 in rabbits was found to be between 1200 and 1400 mg/kg.화재위험
Toluene is a flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Toluene vapor forms explosive mixtures with air at concentrations of 1.4 to 6.7% (by volume). Hazardous gases produced in fire include carbon monoxide and carbon dioxide. Carbon dioxide and dry chemical extinguishers should be used to fight toluene fires.인화성 및 폭발성
Toluene is a flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Toluene vapor forms explosive mixtures with air at concentrations of 1.4 to 6.7% (by volume). Hazardous gases produced in fire include carbon monoxide and carbon dioxide. Carbon dioxide and dry chemical extinguishers should be used to fight toluene fires.화학 반응
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.잠재적 노출
Toluene is used as an industrial chemical, chemical intermediate; solvent, and emulsifier; may be encountered in the manufacture of benzene. It is also used as a chemical feed for toluene diisocyanate, phenol, benzyl and benzoyl derivatives; benzoic acid; toluene sulfonates; nitrotoluenes, vinyltoluene, and saccharin; as a solvent for paints and coatings; or as a component of automobile and aviation fuels.Carcinogenicity
The IARC has determined that there is evidence for the lack of carcinogenicity of toluene in experimental animals and that there is inadequate evidence for carcinogenicity in humans. Results of in vitro assays generally indicate that toluene is not genotoxic. Reports of increased incidences of sister chromatid exchanges and chromatid breaks in exposed workers are confounded by concurrent exposure to other organic chemicals.저장
toluene should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.운송 방법
UN1294 Toluene, Hazard Class: 3; Labels: 3-Flammable liquid.비 호환성
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Violent reaction with mixtures of nitric and sulfuric acid.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.톨루엔 준비 용품 및 원자재
원자재
다이에틸렌 글리콜
백금분
Motor benzol
heavy benzol
light benzol
콜타르 증류액
Petroleum toluene
석유벤진
벤젠
COUMARONE RESIN
수산화나트륨
폴리아크릴산
코크스
황산
준비 용품
Chloroprene-phenolic adhesive 801
3-Amino-3-azabicyclo[3.3.0]octane hydrochloride
QUINOLINE-2-CARBONYL CHLORIDE
2-Thienyl isocyanate
FLUORESCEIN CHLORIDE
(R)-(-)-글리시딜 부티르산염
1-(2-CHLOROBENZYL)PIPERAZINE
5-BROMOPYRIDINE-2-CARBONYL CHLORIDE
디에틸렌글리콜모노노르말헥실에테르
Asphalt antifouling paint L40-32
Methyl 4-oxotetrahydrothiophene-3-carboxylate
(Methoxymethyl)triphenylphosphonium chloride
1,2-DIAMINO-2-METHYLPROPANE
N-(Chloromethyl)phthalimide
트리스노닐페닐 아인산염
디클로로 비스 (벤조 니트릴) 팔라듐 (II)
1,3-DIMETHYL-2-(2-FURYL)IMIDAZOLIDINE
1-Butyl-3-methylimidazolium bromide
안티모니 트라이아세트산(안티몬 트리아세트산)
1-(3-CHLOROBENZYL)PIPERAZINE
3-Thiophenecarbonyl chloride
2-AMINO-4-METHOXYPYRIMIDINE
3-Cyanophenyl isocyanate
디클로로(1,5-시클로옥타딘)팔라디움(II)
6-FLUORO-4-HYDROXY-2-(TRIFLUOROMETHYL)QUINOLINE
4-AMINO-6-CHLORO-PYRIMIDINE-5-CARBALDEHYDE
트리메틸아민옥사이드
(11AR)-(+)-10,11,12,13-TETRAHYDRODIINDENO[7,1-DE:1',7'-FG][1,3,2]DIOXAPHOSPHOCIN-5-DIMETHYLAMINE
페닐레프린 수화염화물
N,O-비스(트리메틸시릴)아세트아마이드
5-Methoxypyridine-3-boronic acid
2-ISOCYANATO-4,6-DIMETHOXYPYRIMIDINE
Chromocene
1-(2,6-DIMETHYLPHENYL)PIPERAZINE
5-ACETYLAMINO-2-CHLORO-4-PICOLINE
2,4-DI-(TERT-BUTOXY)-5-BROMOPYRIMIDINE
2-ETHOXYPYRIMIDIN-4-YLAMINE
2,2-DIMETHYLCYCLOHEXANONE
3,4-(METHYLENEDIOXY)PHENYL ISOCYANATE
헵틸파라벤