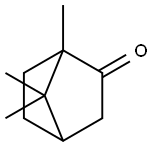
캄파(인조)
|
|
캄파(인조) 속성
- 녹는점
- 175-177 °C(lit.)
- 끓는 점
- 204 °C(lit.)
- 밀도
- 0.992
- 증기 밀도
- 5.2 (vs air)
- 증기압
- 4 mm Hg ( 70 °C)
- 굴절률
- 1.5462 (estimate)
- FEMA
- 4513 | dl-CAMPHOR
- 인화점
- 148 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 아세톤, 에탄올, 디에틸에테르, 클로로포름 및 아세트산에 용해됩니다.
- 색상
- 흰색 또는 무색
- 냄새
- 디프로필렌 글리콜 중 10.00%. 녹나무 같은
- ?? ??
- 녹나무 같은
- 폭발한계
- 0.6-4.5%(V)
- optical activity
- [α]20/D +0.15 to -0.15°, c = 10% in ethanol
- 수용성
- 0.12g/100mL(25℃)
- Merck
- 14,1732
- JECFA Number
- 2199
- BRN
- 1907611
- Henry's Law Constant
- (x 10-5 atm?m3/mol): 3.00 at 20 °C (approximate - calculated from water solubility and vapor pressure)
- 노출 한도
- TLV-TWA 12 mg/m3 (2 ppm), STEL 18 mg/m3 (3 ppm) (ACGIH); IDLH 200 mg/m3 (NIOSH). .
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제, 금속염, 가연성 물질, 유기물과 호환되지 않습니다.
- InChIKey
- DSSYKIVIOFKYAU-MHPPCMCBSA-N
- LogP
- 2.38
- CAS 데이터베이스
- 76-22-2(CAS DataBase Reference)
- NIST
- Camphor(76-22-2)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-22-36/37/38-20/21/22 | ||
| 안전지침서 | 16-26-37/39 | ||
| 유엔번호(UN No.) | UN 2717 4.1/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | EX1225000 | ||
| 자연 발화 온도 | 870 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 4.1 | ||
| 포장분류 | III | ||
| HS 번호 | 29142910 | ||
| 유해 물질 데이터 | 76-22-2(Hazardous Substances Data) | ||
| 독성 | LD50 orally in mice: 1.3 g/kg (PB293505) | ||
| IDLA | 200 mg/m3 | ||
| 기존화학 물질 | KE-34423 |
캄파(인조) C화학적 특성, 용도, 생산
용도
훈향,향장품,의류의방충제.개요
Camphor was recorded in the ancient books of traditional Chinese medicine, such as Pin Hui Jing Yao, Ben Cao Gang Mu, and Sheng Lian Fang. There has been a long history for traditional Chinese medicine to use camphor.화학적 성질
Camphor, C1oH160, also known as d-2-camphanone, Japan camphor, laurel camphor,Formosa camphor,and gumcamphor,is a terpene ketone. It is colourless solid with a characteristic odour that is obtained from the wood and bark of the camphor tree and is soluble in water and alcohol. It has two optically active forms (dextro and levo) and an optically inactive mixture (racemic) of these two forms. Camphor is used in pharmaceuticals,in disinfectants, in explosives,and to harden nitrocellulose plastics.물리적 성질
Colorless to white, flammable granules, crystals or waxy semi-solid with a strong, penetrating, fragrant or aromatic odor. Odor threshold concentration is 0.27 ppm (quoted, Amoore and Hautala, 1983).역사
The research and development process of camphor has gone through from the natural product extraction to the modern chemical drug synthesis. For a long time, the Chinese extracted camphor mainly from camphor tree (Cinnamomum camphora), root bark of bodinier cinnamon, and Yunnan camphor tree. With the development of chemical industry, human beings started to use chemical synthesis methods to obtain a large amount of camphor. At present, the chemical synthesis process of camphor in China has been well developed. The synthetic camphor is divided into industrial and pharmaceutical grades. The industrial grade camphor has a content of up to 96% or higher, and the pharmaceutical grade camphor with high purity can meet the standard of pharmacopeia.용도
camphor (Cinnamomum camphora) is credited with anesthetic, antiinflammatory, antiseptic, astringent, cooling, and refreshing properties, and thought to be slightly stimulating to blood circulation and function. once absorbed by the subcutaneous tissue, it combines in the body with glucoronic acid and is released through the urine. Camphor is effective for oily and acne skin treatment, and has a scent similar to eucalyptus. In high concentrations, it can be an irritant and numb the peripheral sensory nerves. natural camphor is derived from an evergreen tree indigenous to Asia, although now its synthetic substitute is often used.Indications
Camphor is a ketone which, when applied in 1% to 3% concentration, has mild antipruritic effects through its anesthetic and counterirritant properties. Counterirritants are substances that, by inducing other sensations such as coolness or warmth, ‘‘crowd out’’ the perception of pain or itch. Camphor is used in various OTC topical analgesic products in concentrations as high as 9%.정의
A ketone occurring naturally in the wood of the cam- phor tree (Cinnamomum camphora).World Health Organization (WHO)
Camphor, an aromatic crystalline substance with mild local anaesthetic activity, is available in preparations for both external application and inhalation. The use of such preparations has precipitated convulsions in susceptible infants. This has led several regulatory authorities to require the inclusion of appropriate warnings on labelling.일반 설명
A colorless or white colored crystalline powder with a strong mothball-like odor. About the same density as water. Emits flammable vapors above 150°F. Used to make moth proofings, pharmaceuticals, and flavorings.공기와 물의 반응
Highly flammable. Slightly soluble in water.반응 프로필
Naphthalene, Camphor, glycerol, or turpentine will react violently with chromic anhydride [Haz. Chem. Data 1967 p. 68].위험도
Evolves flammable and explosive vapors when heated. Eye and upper respiratory tract irri- tant, and anosmia. Questionable carcinogen.건강위험
Vapors of camphor can irritate the eyes, nose,and throat. In humans, such irritation may be felt at >3 ppm concentration. Prolongedexposure can cause headache, dizziness, andloss of sense of smell. Ingestion can causeheadache, nausea, vomiting, and diarrhea,and at high dosages can lead to convulsion,dyspnea, and coma. High dosages can beharmful to gastrointestinal tracts, kidney,and brain.LD50 value, intraperitoneal (mice): 3000mg/kg.
화재위험
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.Clinical Use
Camphor is mainly used for the treatment of pruritic skin diseases, fibrous tissue inflammation, neuralgia, and influenza.Safety Profile
A human poison by ingestion and possibly other routes. An experimental poison by inhalation, subcutaneous, and intraperitoneal routes. A local irritant. Ingestion causes nausea, vomiting, dizziness, excitation, and convulsions. Mutation data reported. UsedToxicology
Camphor is a very toxic compound which can prove fatal for infants and children on ingestion even in very small doses. Camphor products are toxic and especially dangerous to young children. Mouthing or eating camphor can cause seizures. Applying balms or ointments in large amounts and adding it to the water of a room humidifier may also cause children to seize. The onset of symptoms may occur very quickly - as early as 5 to 20 minutes.잠재적 노출
Camphor, a natural product, is used as a plasticizer for cellulose esters and ethers; it is used in lacquers and varnishes; and in explosives and pyrotechnics formulations. It is used as a moth repellent and as a medicinal.Carcinogenicity
Camphor was not teratogenic to rats or rabbits when administered orally during the fetal period of organogenesis at doses up to 1000mg/kg body weight (bw)/day or 681mg/kg bw/day, respectively.9 Signs of maternal toxicity included clonic convulsions, reduced motility, and reduced body weight gain in rats and reduced food consumption and body weight gain in rabbits.운송 방법
UN2717 Camphor, synthetic, Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN1130 camphor oil, Hazard Class: 3; Labels: 3-Flammable liquid비 호환성
May form explosive mixture with air. Violent, possibly explosive, reaction with strong oxidizers, especially chromic anhydride, potassium permanganate. May accumulate static electrical charges, and may cause ignition of its vapors.폐기물 처리
ncineration of a solution in a flammable solven.캄파(인조) 준비 용품 및 원자재
원자재
자일렌
CRESYL VIOLET ACETATE
Glass-lined reaction pot
Metatitanic acid
테레핀유
C. I. Pigment Blue 30 (77420)
이소보르네올
황산동(II)
아세트산(빙초산)
수산화나트륨
캠포 기름
수산화칼슘
탄산나트륨(경회)
준비 용품
캄파(인조) 공급 업체
글로벌( 633)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Lewoo Pharmatech(Shanghai)Co.,Ltd | +86-15800805830 +86-15800805830 |
miao@lewoopharma.com.cn | China | 156 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 6011 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 |
kyra@quanjinci.com | China | 1534 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 958 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 3001 | 58 |
| Apeloa production Co.,Limited | +8619933239880 |
admin@apcl.com.cn | China | 341 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 2999 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18032476855 +86-18032476855 |
admin@hblongbang.com | China | 964 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |