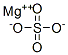황산마그네슘
|
|
황산마그네슘 속성
- 녹는점
- 1124 °C
- 밀도
- 1.07 g/mL at 20 °C
- 증기 밀도
- <0.01 (vs air)
- 증기압
- <0.1 mm Hg ( 20 °C)
- 저장 조건
- no restrictions.
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 물리적 상태
- 가루 (아주 미세한)
- 색상
- 약간 회색
- Specific Gravity
- 2.66
- 수소이온지수(pH)
- 7.9 (50g/l, H2O, 25℃)
- 냄새
- 100.00%에서. 냄새 없는
- ?? ??
- 냄새 없는
- 수용성
- 물에 용해됩니다. 알코올, 글리세롤에 약간 용해됩니다. 아세톤에 불용성.
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.03
λ: 280 nm Amax: 0.02
- 감도
- Hygroscopic
- Merck
- 14,5691
- Dielectric constant
- 8.2(Ambient)
- 안정성
- 안정적인. 흡습성.
- LogP
- -1.031 (est)
- CAS 데이터베이스
- 7487-88-9(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 22-24/25-36-26 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | OM4500000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 28332100 | ||
| 유해 물질 데이터 | 7487-88-9(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-22752 |
황산마그네슘 C화학적 특성, 용도, 생산
물성
수용성의 쓴맛이 나는 무색 결정. MgSO4. 무수염 외에 1, 2, 4, 5, 6, 7 및 12 수화물이 알려져 있다. 가장 보편적인 것은 7수화물이며 사리염 또는 엡솜염이라 불린다. 오래 전부터 완화제로 알려져 있다. 종이의 충전제, 내화제, 매염제, 의약품, 마그네슘 비료로 사용된다.용도
1) 공업적으로는 해수 또는 칼리 공업에서 부차 생성된다. 독일에서는 칼륨염 채취시 결정하는 키제르석을 뜨거운 물에 녹이고 용액을 정치 냉각하여 7수화염을 결정화시키고 있다. 2) 산화마그네슘 또는 히드록시탄산마그네슘을 황산에 녹인 후 미량으로 존재하는 중금속 불순물을 제거하기 위해 마그네슘염을 약간 과잉으로 가해 가열해서 거른다. 모액을 다시 농축하여 공랭하면 7수화염이 결정화한다. 이것을 걸러, 분리하여 자연 건조한다. 3) 무수염은 함수염을 적열 탈수하여 얻어진다.용도
종이의 충전제, 비단의 증가량제, 내화 목면천의 제조, 매염제, 의약으로서 이용된다. 6국 기재의 의약품 : MgSO4ㆍ7H2O. 강열할 때 MgSO4로서 99.5% 이상 함유. 염류 하제로서 내복. 용량 : 1회 8g, 1일 15g. 경련 진정, 진통, 마취 목적에서는 주사제로서 보통 1회 1g을 이용한다.순도시험
(1) 용상 : 이 품목 1g을 물 10mL에 녹일 때, 7수염은 무색으로서 거의 징명 이하이어야 하고, 3수염은 무색으로서 약간 미탁 이하이어야 한다.
(2) 염화물 : 이 품목 1g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.4mL에 대응하는 양 이하이어야 한다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(2.0ppm 이하).
(5) 셀레늄 : 이 품목 0.2g을 정밀히 달아 「황산」의 순도시험 (6)에 따라 시험한다(30ppm 이하).
(6) 철 : 순도시험 (4)의 시험용액을 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 20ppm 이하이어야 한다.
확인시험
이 품목은 확인시험법 중 마그네슘염 및 황산염의 반응을 나타낸다.
정량법
이 품목을 강열한 다음 0.6g을 정밀히 달아 염산(1→4) 2mL 및 물을 가하여 녹이고 정확히 100mL로 한다. 이 액 25mL를 정확히 취하여 물 50mL와 암모니아•염화암모늄완충액(pH 10.7) 5mL를 가하여 0.05M 이.디.티.에이.용액으로 적정한다(지시약 : 에리오크롬블랙T시액 5방울). 종말점은 액의 적자색이 청색으로 변하는 점으로 한다. 따로 같은 방법으로 공시험을 한다.
0.05M 이.디.티.에이.용액 1mL = 6.018mg MgSO4
정의
이 품목에는 결정물(7수염) 및 건조물(3수염)이 있고, 각각을 황산마그네슘(결정) 및 황산마그네슘(건조)라 칭한다.
마그네슘(magnesium) 보충제의
황산마그네슘은 식탁용 소금과 비슷한 질감입니다. 변비 치료제로 먹을 수 있지만, 불쾌한 맛으로 인해 병원에서 주로 정맥주사로 사용되는 경우 많습니다.황산마그네슘은 아픈 근육을 진정시키고 스트레스를 완화시킵니다. 그것은 또한 때때로 로션이나 바디 오일 같은 피부 관리 제품에 포함됩니다.
적절한 마그네슘 수치가 근육 이완과 스트레스 해소에 중요한 역할을 할 수 있지만, 이러한형태가 피부를 통해 잘 흡수된다는 증거는거의 없습니다.
개요
Magnesium sulfate is an inorganic salt (chemical compound) containing magnesium, sulfur and oxygen, with the formula MgSO4. It is strongly hygroscopic and often encountered as the heptahydrate sulfate mineral epsomite (MgSO4•7H2O), commonly called Epsom salt. And the monohydrate, MgSO4•H2O, is found as the mineral kieserite.
In gardening and other agriculture, magnesium sulfate is used to correct a magnesium or sulfur deficiency in soil.
In food preparation, magnesium sulfate is used as a brewing salt in beer production or used as a coagulant for making tofu.
In chemistry, anhydrous magnesium sulfate is commonly used as a desiccant in organic synthesis due to its affinity for water.
For Marine use, magnesium sulfate heptahydrate is used to maintain the magnesium concentration in marine aquaria which contain large amounts of stony corals.
For medicine use, it is used in pregnant women to control seizures due to certain complications of pregnancy (eg, severe toxemia) and to control high blood pressure, severe brain function problems (encephalopathy), and seizures in children who have sudden, severe inflammation of the kidneys (acute nephritis). Besides, magnesium sulfate is also used as a laxative to relieve occasional constipation.
화학적 성질
Magnesium sulfate (MgS04) is a colorless crystal with a bitter, saline taste. It is soluble in glycerol and used in fireproofing, textile processes, ceramics, cosmetics, and fertilizers.물리적 성질
In their hydrated form, these salts have a pH of 6.0 (5.5 to 6.5) in solution. These magnesium sulfates are white crystalline solids. Their densities are: 2.66 g/cm3 (anhydrous); 2.445 g/cm3 (monohydrate); 1.68 g/cm3 (heptahydrate). Solubilities in water are: anhydrous= 26.9 g/100 ml (0°C); monohydrate= 25.5 g/100 ml (20°C); heptahydrate=71 g/100 ml (20°C). Magnesium sulfate is found in nature in many salt deposits and mineral waters, occurring as hydrates or double salts. The heptahydrate or Epsom salt was discovered in 1695, found inthemineralwater at Epsom. Kieserite and epsomite are the two most important minerals. Other than these and the above hydrates, magnesium sulfate is also found in several other minerals, including langbeinite, leonite,vanthoffite,bloedite,kainite,polyhalite,용도
Magnesium sulfate is used widely in several industries including fertilizer, cement, textile, chemicals, and medicine. In the cement industry, it is used in manufacturing oxysulfate cement. In medicine, it is an analgesic and cathartic. An important application of anhydrous magnesium sulfate in the laboratory involves drying organic solvents required for syntheses and GC analysis.In the textile industry, magnesium sulfate is used in finishing composition for dressing cotton; for weighting and sizing silk; as a mordant for fixing basic dyestuffs on wool; and in fireproofing fabrics. It also is a component of certain types of electrolytic plating baths; of various photographic solutions; of cosmetic lotions. It is a catalyst carrier; a dietary supplement in cattle feed; a coagulant for rubber and plastic; and is used in making citric acid and several magnesium salts, such as magnesium stearate.
생산 방법
Magnesium sulfate is widely distributed in nature, e.g. in salt deposits as kieserite, as Epsom salt MgSO4 . 7H20, in the form of double salts such as kainite 4KCl . 4MgSO4 . 11H20 and langbeinite K2SO4 . 2MgSO4, and in brines. Large quantities of kieserite, Epsom salt and anhydrous magnesium sulfate are produced in the processing of potassium salts. Magnesium sulfate is also produced by reacting magnesium carbonate or seawatermagnesium hydroxide with sulfuric acid.제조 방법
Hydrated magnesium sulfate occurs in nature as the minerals kieserite and epsomite. The salt is mined in large scale from these and other naturally occurring minerals. The salt also is prepared in the laboratory by the action of sulfuric acid on magnesium oxide, hydroxide, or carbonate followed by evaporation and crystallization:MgO + H2SO4 → MgSO4 + H2O
Mg(OH)2 + H2SO4 → MgSO4 + 2H2O
MgCO3 + H2SO4 → MgSO4 + CO2 + H2O
Crystallization at temperatures between 1.8 and 48°C yields heptahydrate, MgSO4•7H2O. Below 1.8°C, a dodecahydrate , MgSO4•12H2O crystallizes out. Above 48°C crystals of lower hydrates form. The anhydrous salt is obtained by heating the heptahydrate at about 500°C in a rotary drum; or dehydrating above 150°C in the presence of sulfuric acid.
주요 응용
Magnesium sulfate is utilized in the potassium chemicals industry for the manufacture of potassium sulfate (from potassium chloride), sodium sulfate and potash magnesia (potassium magnesium sulfate). Magnesium sulfate, particularly as kieserite, is used as a fertilizer (ca. 80% of total consumption). It is also used in the textile industry, in the manufacture of building and refractory materials, in the pulp industry and in the production of animal feedstuffs and motor oil additives.정의
ChEBI: A magnesium salt having sulfate as the counterion.Indications
Magnesium sulfate prevents convulsions in preeclampsia and directly uncouples excitation–contraction in myometrial cells through inhibition of cellular action potentials. Furthermore, magnesium sulfate decreases calcium uptake by competing for its binding sites, activating adenylyl cyclase (thereby reducing intracellular calcium), and stimulating calcium-dependent adenosine triphosphatase (ATPase), which promotes calcium uptake by the sarcoplasmic reticulum. Magnesium is filtered by the glomerulus, so patients with low glomerular filtration will have low magnesium clearance. Although the compound does have some cardiac side effects, magnesium sulfate may be preferred over β- adrenergic agents in patients with heart disease, diabetes, hypertension, or hyperthyroidism.Biological Functions
Magnesium sulfate may be effective in terminating refractory ventricular tachyarrhythmias, particularly polymorphic ventricular tachycardia. Digitalis-induced arrhythmias are more likely in the presence of magnesium deficiency. Magnesium sulfate can be administered orally, intramuscularly, or, preferably, intravenously,when a rapid response is intended.The loss of deep tendon reflexes is a sign of overdose.일반 설명
Magnesium sulfate is an anhydrous magnesium salt.농업용
Magnesium sulphate is a white compound existing both in anhydrous (rhombic) and hydrated crystalline forms. The monohydrate MgSO4·H2O (monoclinic) occurs in nature as kieserite. It is a greyish-white crystalline powder which contains about 16 % magnesium and is used as a fertilizer. It is regarded as a concentrated form of epsom salt, having less water of crystallization. The commonest hydrate is heptahydrate MgSO4·7H2O(also called rhombic or epsom salt) which occurs naturally as the mineral epsomite. It is a white powder with a bitter, saline taste. The salt in the monocliic form loses its structural water at 150°C, while the rhombic form loses water at 200°C.Magnesium sulphate is used in sizing and freproofng cotton and silk, in tanning leather, in the manufacture of fertilizers, in explosives and matches, in medicines as a laxative, and as a veterinary medicine for the treatment of inflammations and infected wounds.
Clinical Use
There is much debate as to the efficacy of magnesium sulfate. For effective inhibition of uterine activity, enough must be given to maintain a blood plasma level of at least 5.5 mEq/L. Even at this level, tocolysis may be hard to achieve.부작용
The side effects of magnesium sulfate administration are dose dependent. As magnesium levels increase, skeletal muscle weakness increases and CNS depression and vascular dilation occur. Magnesium sulfate infusion commonly results in a slight decrease in blood pressure during epidural anesthesia.Cardiac muscle is not affected to a clinically evident degree when magnesium is administered at therapeutic levels, although magnesium can have profound myocardial effects during a gross overdose.
Magnesium antagonizes the vasoconstrictive effect of α-agonists, so ephedrine and phenylephrine are likely to less effectively increase maternal blood pressure when administered concomitantly with magnesium.
Magnesium is eliminated unchanged by the kidneys. In a patient who is receiving a maintenance infusion of magnesium and who has decreasing urine output, blood levels of magnesium quickly increase, as do related side effects.
Other side effects of magnesium sulfate include the following: (1) Cutaneous vasodilation with flushing (2) Headache and dizziness (3) Nausea (4) Skeletal muscle weakness (5) Depression of deep tendon reflexes (6) Respiratory depression (7) ECG changes
Safety Profile
A poison by intravenous route. Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. Human systemic effects: heart changes, cyanosis, flaccid paralysis with appropriate anesthesia. An experimental teratogen. Mutation data reported. Potentially explosive reaction when heated with ethoxyethynyl alcohols (e.g., l-ethoxy 3-methyl-1-butyn-3-01). When heated to decomposition it emits toxic fumes of SOx. See also SULFATES.Purification Methods
Crystallise it from warm H2O (1g/mL) by cooling. Dry the heptahydrate (Epsom salt) at ~250o until it loses 25% of its weight. Its solubility in H2O is 36% at 20o, 55% at 60o and 74% at 100o; above 110o the solubility decreases with rise of temperature. Store it in a sealed container.주의 사항
Magnesium sulfate is not for use in patients with heart block or extensive myocardial damage. Use it with caution in patients with impaired renal function, in digitalized patients, and with concomitant use of other central nervous system depressants or neuromuscular blocking agents. Intravenous administration is contraindicated during the 2 hours preceding delivery. Oral administration is contraindicated in patients with abdominal pain, nausea, vomiting, fecal impaction, or intestinal irritation, obstruction, or perforation.참고 문헌
1. https://en.wikipedia.org/wiki/Magnesium_sulfate2. https://www.drugs.com/cdi/magnesium-sulfate.html
3. https://pubchem.ncbi.nlm.nih.gov/compound/magnesium_sulfate
4. http://www.emedicinehealth.com/drug-magnesium_sulfate/article_em.htm
황산마그네슘 준비 용품 및 원자재
원자재
준비 용품
황산마그네슘 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3050 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 |
sales01@sdlookchemical.com | China | 2739 | 58 |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 |
aroma@qdtrustagri.com | China | 165 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 43348 | 58 |
| Aladdin Scientific | +1-833-552-7181 |
sales@aladdinsci.com | United States | 57511 | 58 |
| Aladdin Scientific | +1-833-552-7181 |
sales@aladdinsci.com | United States | 52927 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 |
admin01@hsnm.com.cn | China | 2118 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 18229 | 58 |