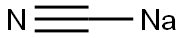Sodium cyanide suppliers
Sodium cyanide
- CAS:
- 143-33-9
- MF:
- CNNa
- MW:
- 49.01
Properties
- Melting point:
- 563.7 °C(lit.)
- Boiling point:
- 1497°C
- Density
- 1.6
- vapor density
- 1.7 (vs air)
- vapor pressure
- 1 mm Hg ( 817 °C)
- Flash point:
- 1500°C
- storage temp.
- Poison room
- solubility
- H2O: 1 M at 20 °C, clear, colorless
- form
- Solid
- pka
- 9.36[at 20 ℃]
- color
- White
- Odor
- The dry salts are odorless, but reaction with atmospheric moisture produces HCN, whose bitter almond odor is detectable at 1 to 5 ppm; however, 20 to 60% of the population are reported to be unable to detect the odor of HCN.
- PH
- 11.7 (100g/l, H2O, 20°C)
- PH Range
- 11-12
- Water Solubility
- 37 g/100mL (20 ºC)
- Sensitive
- Hygroscopic
- Merck
- 14,8605
- BRN
- 3587243
- Exposure limits
- TLV-TWA (measured as CN) skin 5 mg CN/m3 (ACGIH and OSHA), 5 mg CN/m3/ 10-minute ceiling (NIOSH).
- Dielectric constant
- 7.6(Ambient)
- Stability:
- hygroscopic
- LogP
- -0.25 at 20℃
Safety Information
- Symbol(GHS)




GHS05,GHS06,GHS08,GHS09
- Signal word
- Danger
- Hazard statements
- H290-H300+H310+H330-H372-H400
- Precautionary statements
- P262-P273-P280-P302+P352+P310-P304+P340+P310-P314
- Hazard Codes
- T+,N
- Risk Statements
- 26/27/28-32-50/53-48/25
- Safety Statements
- 7-28-29-45-60-61-28A
- RIDADR
- UN 1689 6.1/PG 1
- WGK Germany
- 3
- RTECS
- VZ7525000
- TSCA
- Yes
- HazardClass
- 6.1
- PackingGroup
- I
- HS Code
- 28371110
- Toxicity
- LD50 orally in rats: 15 mg/kg (Smyth)
Use
Sodium cyanide is a white crystalline solid that is odourless when dry but emits a slight odour of HCN in damp air. It is slightly soluble in ethanol and formamide. It is very poisonous. It explodes if melted with nitrite or chlorate at about 450°F. It produces a violent reaction with magnesium, nitrites, nitrates, and nitric acid. On contact with acid, acid fumes, water, or steam, it will produce toxic and flammable vapours.for the extraction of gold and silver ore, copper, zinc, carburizing, medicine and so on. For metallurgy, steel quenching, electroplating, extraction (forming cyanide), organic synthesis of raw materials, insecticidal and anti-corrosion.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "剧毒、有毒、易致癌化学品". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660