| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
| Company Name: |
2A PharmaChem USA
|
| Tel: |
(630) 737-0988 |
| Email: |
sales@2apharmachem.com |
| Products Intro: |
|
|
| | hexobendine Basic information |
| Product Name: | hexobendine | | Synonyms: | hexobendine;Hexabendin;Hexabendine;Hexobendin;N,N'-Dimethyl-N,N'-bis[3-(3,4,5-trimethoxybenzoyloxy)propyl]ethylenediamine;3,4,5-trimethoxybenzoic acid 3-[methyl-[2-[methyl-[3-(3,4,5-trimethoxybenzoyl)oxypropyl]amino]ethyl]amino]propyl ester;3-[methyl-[2-[methyl-[3-(3,4,5-trimethoxybenzoyl)oxypropyl]amino]ethyl]amino]propyl 3,4,5-trimethoxybenzoate;3-[methyl-[2-[methyl-[3-(3,4,5-trimethoxyphenyl)carbonyloxypropyl]amino]ethyl]amino]propyl 3,4,5-trimethoxybenzoate | | CAS: | 54-03-5 | | MF: | C30H44N2O10 | | MW: | 592.68 | | EINECS: | 200-189-1 | | Product Categories: | | | Mol File: | 54-03-5.mol | 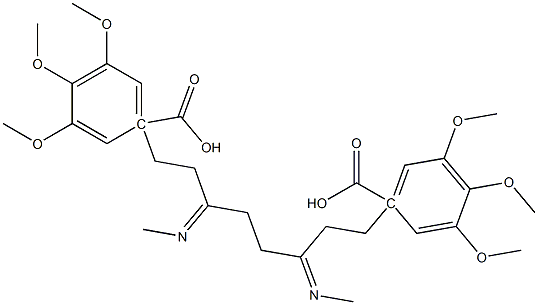 |
| | hexobendine Chemical Properties |
| Melting point | 75-77° | | pka | pKa 4.52 ± 0.01;8.47 ± 0.01(H2O,t = 25.0±0.1,I=0.1,N2)(Approximate) | | CAS DataBase Reference | 54-03-5 |
| | hexobendine Usage And Synthesis |
| Originator | Reoxyl,Hormonchemie,W. Germany,1966 | | Uses | Vasodilator
. | | Definition | ChEBI: Hexobendine is a trihydroxybenzoic acid. | | Manufacturing Process | Methylacrylate and N,N'-dimethylethylenediamine are first reacted and that
product reduced with lithium aluminum hydride to give a compound A.
To a solution of 13 parts of compound A and 12 parts by volume of absolute
pyridine in 80 parts by volume of absolute dioxane there are added dropwise
and under constant stirring 35 parts of 3,4,5-trimethoxybenzoyl chloride
dissolved in 70 parts by volume of absolute dioxane in the course of 30
minutes. The mixture is stirred for a further 3 hours at a temperature of
100°C and the excess solvent is then evaporated in vacuo. The residue of the
evaporation is treated with ethyl acetate and saturated sodium carbonate
solution, whereafter the organic phase is separated, treated with water, dried
with sodium sulfate and the solvent is removed in vacuo. The residue thus
obtained is taken up in ether and separated from 4 parts of insoluble
trimethoxybenzoic acid anhydride by filtration. After evaporation of the ether
there are obtained 32.5 parts of N,N'-dimethyl-N,N'-bis-[3-(3,4,5-
trimethoxybenzoxy)propyl]-ethylene diamine, corresponding to a yield of 86%
of the theoretical. MP: 75°C to 77°C.
The di-tertiary base thus obtained is dissolved in ether and the solution is
saturated with hydrogen chloride gas. After isolation and reprecipitation from
methanol-ether there is obtained the dihydrochloride melting at 170°C to
174°C. | | Therapeutic Function | Vasodilator |
| | hexobendine Preparation Products And Raw materials |
|