| Company Name: |
Cayman Chemical Company
|
| Tel: |
(800) 364-9897 |
| Email: |
cayman@caymanchem.com |
| Products Intro: |
Product Name:Heptabarbital
CAS:509-86-4
|
| Company Name: |
United States Biological
|
| Tel: |
800.520.3011 or 781.639.5092 |
| Email: |
chemicals@usbio.net |
| Products Intro: |
Product Name:Heptabarb
CAS:509-86-4
|
|
| | heptabarb Basic information |
| Product Name: | heptabarb | | Synonyms: | heptabarb;heptabarbital;Heptamal;heptabarbitone;Heptadorm;Medomin;Medomine;Noctyn | | CAS: | 509-86-4 | | MF: | C13H18N2O3 | | MW: | 250.29 | | EINECS: | 208-107-6 | | Product Categories: | | | Mol File: | 509-86-4.mol | 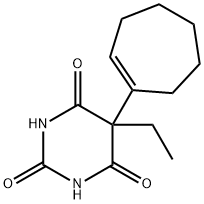 |
| | heptabarb Chemical Properties |
| Melting point | 174° | | Boiling point | 393.43°C (rough estimate) | | density | 1.1307 (rough estimate) | | refractive index | 1.6450 (estimate) | | solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; DMSO:PBS (pH 7.2) (1:3): 0.25 mg/ml; Ethanol: 10 mg/ml | | form | A crystalline solid | | pka | 7.77±0.10(Predicted) | | Water Solubility | 250.3mg/L(25 ºC) |
| RIDADR | 3249 | | HazardClass | 6.1(b) | | PackingGroup | III |
| | heptabarb Usage And Synthesis |
| Chemical Properties | White, crystalline powder; odorless;
slightly bitter taste.Very sparingly soluble in water; slightly soluble in alcohol; soluble
in alkaline solutions. Forms water-soluble sodium,
magnesium, and calcium salts. | | Originator | Medomine,Ciba Geigy,France,1948 | | Uses | Medicine (sedative). | | Definition | ChEBI: Heptabarbital is a member of barbiturates. | | Manufacturing Process | 112 g of cycloheptanone (suberone) are mixed with 130 g of cyanoacetic acid
methyl ester, 2 g of piperidine are added, and the mixture is heated on the
water bath at 60°C for several hours until no more water separates from the
reaction mixture. The water layer is removed, and the remainder is subjected
to distillation in vacuo. The fraction distilling at 160°C to 175°C under a
pressure of 20 mm is collected separately; it consists of cycloheptenyl�cyanoacetic acid methyl ester. The first fractions can be subjected to a fresh
condensing reaction after addition of more piperidine.
The cycloheptenyl-cyanoacetic acid methyl ester so obtained is a colorless
liquid boiling at 174°C under a pressure of 20 mm.
Into this compound, an ethyl radical is introduced at the same C-atom to
which the cycloheptenyl radical is connected. This is done, for example, in the
following way:
19.3 g of the above ester are added to a solution of 2.3 g of sodium in 40 cc
of absolute ethyl alcohol. To this mixture, 13.0 g of ethyl bromide are
gradually added while cooling, and the reaction mixture is heated under reflux
on a water bath until it has become neutral. The mixture is then taken up in
water, the aqueous layer is separated and the cycloheptenyl-ethyl-cyanoacetic
acid methyl ester so formed distills at 169°C to 170°C under a pressure of 20
mm.
22.1 g of this latter substance are dissolved in a solution of 4.6 g of sodium in
100 cc of absolute ethyl alcohol. 12 g of urea are further added thereto, and
the whole solution is heated to about 80°C for about eight hours. The alcohol
is then distilled off in vacuo, the residue is dissolved in cold water, and from
this solution, C-C-cycloheptenyl-ethyl barbituric acid is obtained by
saponification with diluted hydrochloric acid. The crude product is
recrystallized from diluted ethyl alcohol and forms colorless needles of faintly
bitter taste and melting point 174°C.
The sodium salt of this acid may be prepared by dissolving 2.5 g of the acid in
a solution of 0.23 g of sodium in 20 cc of ethyl alcohol, and the salt forms,
after evaporating the alcohol, a colorless, water-soluble powder. | | Brand name | Medapan;Medomina. | | Therapeutic Function | Hypnotic, Sedative |
| | heptabarb Preparation Products And Raw materials |
|