- 2-Propanoic Acid
-

- $60.00 / 1kg
-
2024-05-14
- CAS:138-22-7
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 5000
- Butyl Lactate
-

- $120.00 / 1kg
-
2024-04-29
- CAS:138-22-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Butyl lactate
-

- $10.00 / 1kg
-
2024-04-23
- CAS:138-22-7
- Min. Order: 1kg
- Purity: 99.6%
- Supply Ability: 100000
Related articles - Uses and Hazards of Butyl lactate
- ?Butyl lactate is a colorless liquid with a mild odor. It is used in making paint, inks, perfumes and dry cleaning fluid, and ....
- Dec 30,2022
|
| Product Name: | Butyl lactate | | Synonyms: | FEMA 2205;BUTYL 2-HYDROXYPROPANOATE;BUTYL 2-HYDROXYPROPIONATE;BUTYL LACTATE;Butyl α-hydroxypropionate;N-BUTYL LACTATE;Butyl2-hydroxypropionicacid;butylalpha-hydroxypropionate | | CAS: | 138-22-7 | | MF: | C7H14O3 | | MW: | 146.18 | | EINECS: | 205-316-4 | | Product Categories: | C6 to C7;Carbonyl Compounds;Esters | | Mol File: | 138-22-7.mol | 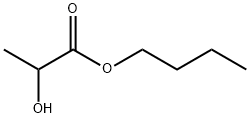 |
| | Butyl lactate Chemical Properties |
| Melting point | -28 °C (lit.) | | Boiling point | 185-187 °C (lit.) | | density | 0.984 g/mL at 25 °C (lit.) | | vapor density | 5.04 (vs air) | | vapor pressure | 0.4 mm Hg ( 20 °C) | | FEMA | 2205 | BUTYL LACTATE | | refractive index | n20/D 1.421(lit.) | | Fp | 157 °F | | storage temp. | Sealed in dry,Room Temperature | | pka | 13.06±0.20(Predicted) | | form | Liquid | | color | Clear colorless | | Odor | at 100.00 %. green fruity apple kiwi melon rind lactonic waxy winey apple skin soapy | | Odor Type | green | | Water Solubility | 42 g/L (25 ºC) | | JECFA Number | 932 | | LogP | 0.88 | | CAS DataBase Reference | 138-22-7(CAS DataBase Reference) | | NIST Chemistry Reference | Propanoic acid, 2-hydroxy-, butyl ester(138-22-7) | | EPA Substance Registry System | n-Butyl lactate (138-22-7) |
| | Butyl lactate Usage And Synthesis |
| Description | Butyl lactate is a kind of lactate derived ester. As a kind of alpha-hydroxy acid ester, its applications in cosmetic, food and pharmaceutical formulations have significantly increased due to their hygroscopic, emulsifying and exfoliating properties. It is used as a food additive because of its flavoring effect. In industry, it can be used as solvent and chemical feedstock. As a bio-based solvent, it can be used as extractant for removing 1-butanol from the aqueous fermentation broths. It can be generally manufactured through the action of lipase from various origins.
| | References | [1]Zheng, Shaohua, et al. "Feasibility of bio-based lactate esters as extractant for biobutanol recovery:(Liquid+ liquid) equilibria." The Journal of Chemical Thermodynamics 93 (2016): 127-131.
[2]Pirozzi, Domenico, and Guido Greco. "Activity and stability of lipases in the synthesis of butyl lactate." Enzyme and microbial technology 34.2 (2004): 94-100.
[3]Koutinas, Athanasios, et al. "Economic evaluation of technology for a new generation biofuel production using wastes." Bioresource technology 200 (2016): 178-185.
| | Description | Butyl lactate is a liquid. Molecularweight = 146.19; Boiling point = 170℃ at 760 mmHg;Freezing/Melting Point = -43℃; Flash point= 71℃(oc). Autoignition temperature =340-382℃. HazardIdentification (based on NFPA-704 M Rating System):Health 1, Flammability 2, Reactivity 0. Slightly soluble inwater. | | Chemical Properties | CLEAR COLOURLESS LIQUID | | Chemical Properties | Butyl lactate has a faintly sweet, pleasant odor with buttery, creamy, milky, sweet, mushroom undertones. Two optically
active and one racemic form of butyl lactate are known. | | Occurrence | Reported found in cognac, cider and white wine. | | Uses | n-Butyl lactate is used as a food and feed additive. | | Uses | Butyl lactate has been used:
- in the preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique
- in the synthesis of nanoparticles of griseofulvin from water dilutable microemulsions by the solvent diffusion technique
- as cosurfactant on the preparation of microemulsions with anionic surfactant
| | Uses | Solvent for nitrocellulose, ethyl cellulose, oils,
dyes, natural gums, many synthetic polymers, lac-
quers, varnishes, inks, stencil pastes, antiskin-
ning agent, chemical (intermediate), perfumes, dry-
cleaning fluids, adhesives. | | Definition | ChEBI: Butyl lactate is a carboxylic ester. | | Preparation | The racemic d-form is prepared by reacting zinc ammonium l-lactate with n-butyl alcohol in the presence of concentrated
H2SO4; the l-form is prepared by reacting zinc ammonium d-lactate with n-butyl alcohol in the presence of HCl; the racemic form
is prepared by several methods, one being from calcium or sodium lactate and n-butyl alcohol in benzene in the presence of H2SO4,
with subsequent azeotropic distillation of the mixture. | | Production Methods | n-Butyl lactate may be prepared via esterification of lactic
acid and n-butyl alcohol. | | Taste threshold values | Taste characteristic at 100 ppm: harsh and sulfuraceous with fruit notes. | | General Description | A clear colorless liquid with a mild odor. Flash point 168°F. Less dense than water and insoluble in water. Vapors heavier than air. Used as a solvent, and to make other chemicals. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Butyl lactate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Avoid contact with strong oxidizing agents and strong bases. Will not polymerize [USCG, 1999]. | | Hazard | Toxic. Upper respiratory tract irritant. | | Health Hazard | VAPOR: Headache, coughing, possible sleepiness, nausea or vomiting, or dizziness may result. LIQUID: Irritating to skin and eyes. | | Safety Profile | Poison by
intraperitoneal route. A skin irritant. Toxic
concentration in air for humans is about 4
ppm. Flammable when exposed to heat or
flame; can react with oxidizing materials. To
fight fire, use alcohol foam, foam, CO2, dry
chemical. When heated to decomposition it
emits acrid smoke and irritating fumes. See
also ESTERS, n-BUTYL ALCOHOL, and
LACTIC ACID. | | Potential Exposure | Butyl lactate is a liquid. Molecular
weight 5 146.19; boiling point 5 170�C @ 760 mmHg;
freezing/melting point 5 243�C; flash point 5 71�C(oc). Autoignition temperature 5 340�382�C. Hazard
identification (based on NFPA-704 M Rating System):
Health 1; flammability 2; reactivity 0 ?. Slightly soluble in
water. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | | storage | Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where butyl lactate may be present, check to makesure that an explosive concentration does not exist. Store intightly closed containers in a cool, well-ventilated area.Metal containers involving the transfer of this chemicalshould be grounded and bonded. Where possible, automatically pump liquid from drums or other storage containers toprocess containers. Drums must be equipped with self-closing valves, pressure vacuum bungs, and flame arresters. Useonly nonsparking tools and equipment, especially whenopening and closing containers of this chemical. Sources ofignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard. | | Shipping | A UN1993 Flammable liquids, n.o.s., Hazard
Class: 3; Labels: 3—Flammable liquid, Technical Name
Required. | | Incompatibilities | May form explosive mixture with air.
Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong bases, strong acids, oxoacids, epoxides | | Waste Disposal | Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. |
| | Butyl lactate Preparation Products And Raw materials |
|