|
|
| | Ethyl (trimethylsilyl)acetate Basic information |
| | Ethyl (trimethylsilyl)acetate Chemical Properties |
| Boiling point | 156-159 °C (lit.) | | density | 0.876 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.415(lit.) | | Fp | 95 °F | | storage temp. | Inert atmosphere,Room Temperature | | solubility | sol ethereal and chlorinated solvents; reacts with
protic solvents. | | form | clear liquid | | color | Colorless to Almost colorless | | Specific Gravity | 0.876 | | Water Solubility | Decomposition | | Hydrolytic Sensitivity | 2: reacts with aqueous acid | | Sensitive | Moisture Sensitive | | BRN | 1755902 | | CAS DataBase Reference | 4071-88-9(CAS DataBase Reference) | | NIST Chemistry Reference | Ethyl (trimethylsilyl)acetate(4071-88-9) |
| Risk Statements | 10 | | Safety Statements | 16-24/25 | | RIDADR | UN 3272 3/PG 3 | | WGK Germany | 3 | | F | 10-21 | | TSCA | Yes | | HazardClass | 3 | | PackingGroup | III | | HS Code | 29319090 |
| | Ethyl (trimethylsilyl)acetate Usage And Synthesis |
| Chemical Properties | Ethyl trimethylsilylacetate is a clear colorless liquid. It is stable to the usual manipulations, and can be stored in glass containers for years without change of physical and spectral properties. | | Uses | Ethyl trimethylsilylacetate is used in the synthesis of ethyl-2,2-dibromo-2-(trimethylsilyl)acetate and α,β-unsaturated esters. It was also used to silylate the enolizable aldehydes and ketones. | | Uses | (silylating agent; source of an ethyl acetate anion equivalent.Ethyl trimethylsilylacetate is reactive to nucleophiles and readily undergoes desilylation reactions with acid or alkali,ethanol, and bromine.
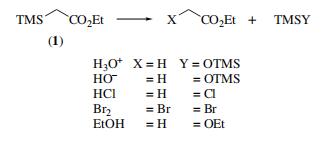 | | Preparation | ethyl trimethylsilylacetate synthesis: Available by a Reformatsky reaction from ethyl bromoacetate, and by reaction of trimethylsilylmethyl�magnesium chloride with ethyl chloroformate. An alternative approach requires the treatment of ethyl acetate with triphenylmethylsodium followed by chlorotrimethylsilane The use of a nitrogen base with ethyl acetate in THF followed by reaction with chlorotrimethylsilane results in a mixture of C- and O-silylation. The use of HMPA as additive in the reaction medium increases the amount of O-silylation to 90%. Similar methods can be used to prepare analogs. | | General Description | Reaction of ethyl trimethylsilylacetate (ETSA) with dilute hydrochloric acid, dilute alkali, anhydrous HCl, anhydrous bromine and absolute ethanol has been reported. ETSA undergoes condensation with aromatic aldehydes in the presence of base catalyst to yield β-trimethylsiloxycarboxylates. Lithium ETSA reacts with ketones to yield α,β-unsaturated esters. | | Purification Methods | Purify it by distilling ca 10g of reagent through a 15cm, Vigreux column (p 11) and then redistilling it through a 21cm glass helices-packed column [Hauze & Hauser J Am Chem Soc 75 994 1953]. Alternatively, dissolve it in Et2O, wash with H2O, dilute Na2CO3, dry over Na2CO3, evaporate Et2O, and distil it through a column of 15 theoretical plates. [Gold et al. J Am Chem Soc 70 2874 1948, Beilstein 4 IV 3974.] |
| | Ethyl (trimethylsilyl)acetate Preparation Products And Raw materials |
|