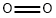Storage and manufacture of Oxygen
Feb 21,2022
Oxygen comprises 21% of the Earth's atmosphere and is the third most abundant element in the universe by mass after hydrogen and helium. It is a highly reactive non-metal with the atomic number 8 and chemical symbol O.
Uses
1. Management of cellular hypoxia (either caused by hypoxaemia or low cardiac output states). 2. Treatment of carbon monoxide poisoning. 3. Treatment of anaerobic infections. 4. Treatment of decompression sickness.
Presentation and storage
Oxygen is a colourless, tasteless and odourless gas at room temperature and pressure. It supports combustion and, in the correct circumstances, is explosive. It has a specific gravity of 1.105 and a molecular mass 32. At atmospheric pressure, it liquefies at −183°C.
There are two common means of oxygen storage for hospital use:
1. Cylinders. Traditionally cylinders containing oxygen have a black body with white shoulders, although there are now lightweight, fibreglass cylinders in use that are fully white in colour. Both types of cylinders store oxygen at a pressure of 137 bar (13,700 kPa) at 15°C when full.
2. Vacuum insulated evaporator (VIE). The VIE is a large vessel that stores liquid oxygen at approximately −160°C and 7 bar and is used in medical or industrial facilities where oxygen demand is high (usually in excess of 150,000 l week −1 ). Oxygen is delivered to the hospital from either the VIE or a cylinder manifold through PMGV and is regulated to 4 bar.
Manufacture
Oxygen can be manufactured from air in two main ways:
1. Fractional distillation of air. Oxygen is manufactured commercially by fractional distillation of liquid air. Before liquefaction of air, carbon dioxide is removed and liquid oxygen and nitrogen separated by means of their different boiling points (oxygen –183°C; nitrogen – 195°C).
2. Oxygen concentrators. Oxygen concentrators produce oxygen from ambient air by absorption of nitrogen onto types of alumina silicates. Oxygen concentrators are useful both in hospitals and in long-term domestic use in remote areas such as in developing countries and in military surgery. The gas formed by oxygen concentrators contains small quantities of inert gases (e.g. argon); these are harmless but reduce the maximal concentration of oxygen that can be produced.
- Related articles
- Related Qustion
- What is oxygen used for? Is it an element? Mar 4, 2024
Oxygen forms a molecule (O2) of two atoms which is a colorless gas at normal temperatures and pressures.Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium.
- How to determine the polarity of oxygen? Dec 20, 2023
Oxygen is an oxidizing agent that forms oxides with multiple compounds readily. There are many known allotropes of oxygen, out of which O2 is the most stable one.
- Oxygen (O) - Chemical properties, Health and the lack of sufficient Oct 29, 2021
Oxygen is a very prevalent and important element and is necessary for sustaining life on this planet. This element is the third most abundant in mass behind helium and hydrogen in the universe. The diatomic form (O2) is the most common pure
Xenon, in common with nitrous oxide and ketamine, acts by non-competitive inhibition of NMDA receptors in the CNS.....
Feb 21,2022APIMonobenzone, also called 4-(Benzyloxy)phenol and monobenzyl ether of hydroquinone (MBEH) is an organic chemical in the phenol family.....
Feb 21,2022Organic reagentsOxygen
7782-44-7You may like
- The structure of Vanadium carbide
May 11, 2024
- The research on corundum-type Ti2O3
May 10, 2024
- Tungsten nitrides and W-N structures
May 10, 2024
- Oxygen USP/EP/BP
-
- $1.10 / 1g
- 2021-07-29
- CAS:7782-44-7
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min