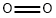what is the atomic number of the element located in group 16, period 2 of the periodic table?
Mar 7,2024
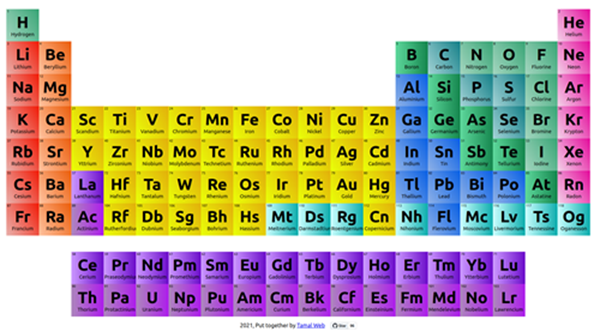
The atomic number of the element in Group 16, Period 2 of the Periodic Table is 8 and the element is oxygen. A horizontal row in the periodic table is called a period. There are only two elements in Period 1: hydrogen and helium. Period 2 has eight elements. Based on the periodic table chart (see below), we can see that the atomic number of the element in the 16th group of the 2nd cycle of the periodic table is 8, which corresponds to the element oxygen.
- Related articles
- Related Qustion
- What is oxygen used for? Is it an element? Mar 4, 2024
Oxygen forms a molecule (O2) of two atoms which is a colorless gas at normal temperatures and pressures.Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium.
- How to determine the polarity of oxygen? Dec 20, 2023
Oxygen is an oxidizing agent that forms oxides with multiple compounds readily. There are many known allotropes of oxygen, out of which O2 is the most stable one.
- Storage and manufacture of Oxygen Feb 21, 2022
Oxygen is a colourless, tasteless and odourless gas at room temperature and pressure. It supports combustion and, in the correct circumstances, is explosive.
Magnesium malate is a chemical compound that consists of magnesium and malic acid, which is a fundamental metabolite.....
Mar 7,2024Organic ChemistrySilver chloride is a white crystalline solid that is well known for its low solubility in water and its sensitivity to light.....
Mar 7,2024Inorganic chemistryOxygen
7782-44-7You may like
- Oxygen USP/EP/BP
-
- $1.10 / 1g
- 2021-07-29
- CAS:7782-44-7
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min