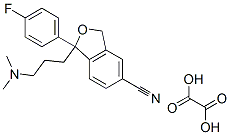
Escitalopram oxalate synthesis
- Product Name:Escitalopram oxalate
- CAS Number:219861-08-2
- Molecular formula:C22H23FN2O5
- Molecular Weight:414.43
144-62-7
741 suppliers
$5.00/5g

128196-01-0
155 suppliers
$40.00/5mg

219861-08-2
534 suppliers
$5.00/100mg
Yield:219861-08-2 98.57%
Reaction Conditions:
in acetone at 25 - 30;Industry scale;
Steps:
2
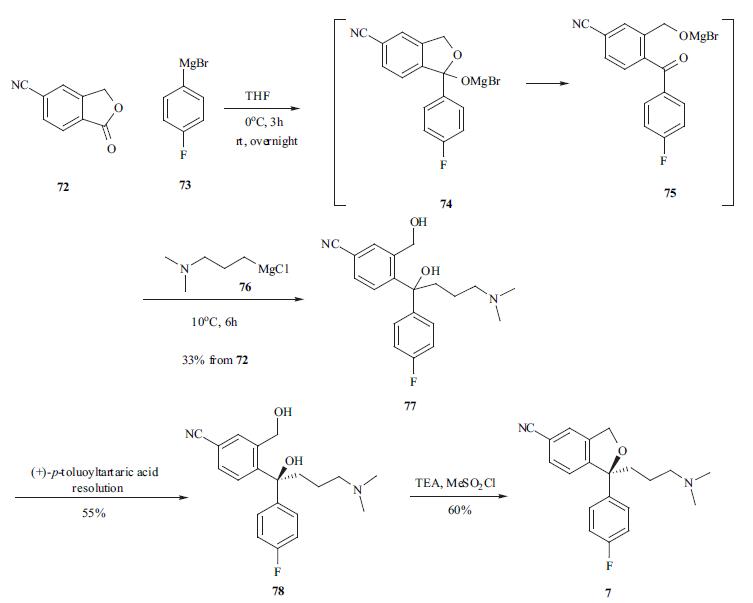
Preparation of Escitalopram OxalateThe residue obtained in Example 1 was dissolved in acetone (120 lit) at 25-30° C. A solution of oxalic acid dihydrate (17 Kg, 0.135 kmoles) in acetone (34 lit) was introduced over 30 minutes. The reaction mass was stirred for 2 hrs, cooled to 10° C. and further stirred for 1 hr. The solid obtained was isolated by filtration, washed with acetone (2×40 lit) and dried in a vacuum oven for 4-5 hrs at 50-55° C. to obtain the title compound.Yield: 40.0 Kg, 98.57%Chiral purity:->99.5%
References:
US2010/204493,2010,A1 Location in patent:Page/Page column 8

144-62-7
741 suppliers
$5.00/5g
![(-)-4-[4-(Dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)benzonitrile](/CAS/GIF/488787-59-3.gif)
488787-59-3
64 suppliers
inquiry

219861-08-2
534 suppliers
$5.00/100mg
![Benzonitrile, 3-[(4-chloro-2-nitrophenoxy)methyl]-4-[(1S)-4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl]-, hydrochloride (1:1)](/CAS/20211123/GIF/878007-22-8.gif)
878007-22-8
0 suppliers
inquiry

144-62-7
741 suppliers
$5.00/5g

219861-08-2
534 suppliers
$5.00/100mg
50-00-0
841 suppliers
$10.00/25g

144-62-7
741 suppliers
$5.00/5g

219861-08-2
534 suppliers
$5.00/100mg
![5-Isobenzofurancarbonitrile, 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-, (1S)-, (2R,3R)-2,3-dihydroxybutanedioate (1:1)](/CAS/20211123/GIF/1073819-95-0.gif)
1073819-95-0
0 suppliers
inquiry

144-62-7
741 suppliers
$5.00/5g

219861-08-2
534 suppliers
$5.00/100mg