| Identification | More | [Name]
Rubidium fluoride | [CAS]
13446-74-7 | [Synonyms]
RUBIDIUM FLUORIDE
RbF
Rubidium fluoride (RbF)
rubidiumfluoride(rbf)
rubidiumfluorideanhydrous
rubidiummonofluoride
RUBIDIUM FLUORIDE, 99.8%
RUBIDIUM FLUORIDE ANHYDROUS (99.8%-RB)
RUBIDIUM FLUORIDE, PURATRONIC, 99.975% (METALS BASIS)
RUBIDIUM FLUORIDE ANHYDROUS (99%-RB)
rubidium fluoride, puratronic
RUBIDIUMFLUORIDE,ULTRAPURE
Rubidium fluoride anhydrous 99%
Rubidiumfluorideanhydrous99%
RUBIDIUM FLUORIDE, ANHYDROUS: 99.8%
Rubidium fluoride, Puratronic(R), 99.975% (metals basis)
Rubidium fluoride, 99.7% (metals basis)
Rubidium fluoride, 99% (metals basis)
Rubidium fluoride(85Rb) | [EINECS(EC#)]
236-603-2 | [Molecular Formula]
FRb | [MDL Number]
MFCD00011191 | [Molecular Weight]
104.47 | [MOL File]
13446-74-7.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [Melting point ]
775°C | [Boiling point ]
1410°C | [density ]
3.557 g/mL at 25 °C(lit.) | [refractive index ]
1.398 | [solubility ]
ethanol: insoluble(lit.) | [form ]
Crystalline | [color ]
White | [Specific Gravity]
3.557 | [Stability:]
Stable. Hygroscopic. Non-flammable, but HF may be released in a fire. Incompatible with acids. | [Water Solubility ]
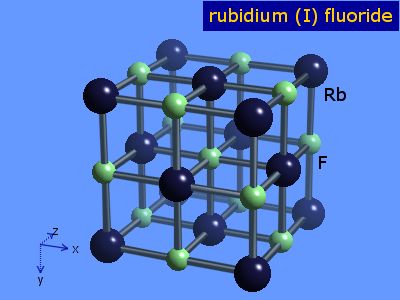
soluble | [Crystal Structure]
NaCl type | [Sensitive ]
Hygroscopic | [crystal system]
Cube | [Space group]
Fm3m | [Lattice constant]
| a/nm | b/nm | c/nm | α/o | β/o | γ/o | V/nm3 | | 0.5652 | 0.5652 | 0.5652 | 90 | 90 | 90 | 0.1805 |
| [Exposure limits]
ACGIH: TWA 2.5 mg/m3
NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 | [InChI]
1S/FH.Rb/h1H;/q;+1/p-1 | [InChIKey]
AHLATJUETSFVIM-UHFFFAOYSA-M | [SMILES]
[F-].[Rb+] | [CAS DataBase Reference]
13446-74-7(CAS DataBase Reference) | [NIST Chemistry Reference]
Rubidium fluoride(13446-74-7) | [EPA Substance Registry System]
13446-74-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R40:Limited evidence of a carcinogenic effect.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S22:Do not breathe dust . | [RIDADR ]
UN3288 | [WGK Germany ]
3 | [RTECS ]
VL8740000 | [Hazard Note ]
Irritant | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
28261990 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Dermal
Acute Tox. 4 Inhalation
Acute Tox. 4 Oral
Carc. 2 | [Safety Profile]
Poison as a soluble fluoride. When heated to decomposition it emits toxic fumes of RbzO and F-. See also RUBIDIUM and FLUORIDES. |
| Hazard Information | Back Directory | [Hazard]
Strong irritant to tissue.
| [Chemical Properties]
Rubidium fluoride is a colorless cubic crystal. It is highly toxic and soluble in water and dilute hydrofluoric acid solutions, but insoluble in ethanol, diethyl ether, and ammonia. Its hydrofluoric acid solution exhibits strong acidity and can corrode glass.
 | [Uses]
As raw materials for preparation of rubidium metal and various rubidium salts, for the manufacturing of catalyst and for the manufacturing of high energy density micro cells and crystal scintillation counters. | [Preparation]
Rubidium fluoride is produced by concentrating the reaction product of rubidium carbonate with hydrofluoric acid. | [Reactions]
There are several methods for synthesising rubidium fluoride. One involves reacting rubidium hydroxide with hydrofluoric acid:
RbOH + HF → RbF + H2O
Another method is to neutralize rubidium carbonate with hydrofluoric acid:
Rb2CO3 + 2HF → 2RbF + H2O + CO2
Another possible method is to react rubidium hydroxide with ammonium fluoride:
RbOH + NH4F → RbF + H2O + NH3
The least used method due to expense of rubidium metal is to react it directly with fluorine gas, as rubidium reacts violently with halogens:
2Rb + F2 → 2RbF |
|
|