| Identification | Back Directory | [Name]
(S,S)-(+)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-COBALT(II) | [CAS]
188264-84-8 | [Synonyms]
(S,S)-(+)-N,N′
(S,S)-(+)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexane
-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminocobalt(II)
(S,S)-N,N'-BIS(3,5-DI-T-BUTYLSALICYLIDEN E) -1,2-CYCLOHEXANEDIAMINOCOBALT(II)
(1S,2S)-(+)-N,N'-BIS(3,5-DI-T-BUTYLSALICYDENE)-1,2-CYCLOHEXANEDIAMINOCOBALT(II)
(1S,2S)-(+)-N,N-Bis(3,5-di-t-butylsalicylidene)-1,2-cyclohexanediaminocobalt(II)
(1S,2S)-(+)-1,2-CYCLOHEXANEDIAMINO-N N'-BIS(3,5-DI-T-BUTYLSALICYLIDENE)COBALT(II)
(1S,2S)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-
cyclohexanediaminocobalt(II)
(S,S)-(+)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-COBALT(II)
(1S,2S)-(+)-1,2-Cyclohexanediamino-N,N'-bis(3,5-di-tert-butylsalicylidene)cobalt(II)
(S,S)-(+)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminocobalt(II),98%
2,2'-{(1S,2S)-1,2-Cyclohexanediylbis[nitrilo(E)methylylidene]}bis [4,6-bis(2-methyl-2-propanyl)phenol] - cobalt (1:1)
Cobalt,[[2,2'-[(1S,2S)-1,2-cyclohexanediylbis[(nitrilo-kN)methylidyne]]bis[4,6-bis(1,1-dimethylethyl)phenolato-kO]](2-)]-, (SP-4-2)- | [Molecular Formula]
C36H52CoN2O2 | [MDL Number]
MFCD01631278 | [MOL File]
188264-84-8.mol | [Molecular Weight]
603.74 |
| Chemical Properties | Back Directory | [Appearance]
red to red-brown powder | [Melting point ]
>350 °C(lit.)
| [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [form ]
Powder | [color ]
red-brown | [InChIKey]
ZFWPDJKMASHRPT-DKZUAMKGNA-L | [SMILES]
C(C1C=C(C(C)(C)C)C=C2C=N3[C@@]4([H])CCCC[C@]4([H])N4=CC5=CC(C(C)(C)C)=CC(C(C)(C)C)=C5[O-][Co+2]43[O-]C=12)(C)(C)C |&1:12,18,r| |
| Hazard Information | Back Directory | [Chemical Properties]
red to red-brown powder | [Uses]
(S,S)-(+)-N,N''-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminocobalt(ii) i sused as a catalyst for the hydrolytic kinetic resolution of terminal epoxides and the enantioselective ring opening of meso epoxides. | [Synthesis]
The general procedure for the synthesis of (S,S)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine cobalt(II) from cobalt acetate and 1,2-cyclohexylamine bis(3,5- butyl sulphate) is as follows: with reference to Example 1, (R,R)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine (10.9 g. 20.0 mmol) was dissolved in dichloromethane (80 mL). The solution was slowly added to a methanol solution of cobalt tetrahydrate acetate (5.98 g, 24.0 mmol) (80 mL) and the reaction was stirred at room temperature for 15 min. A red solid gradually precipitated during the reaction. The reaction mixture was then continued to be stirred at 0 °C for 30 min to promote complete precipitation. The red solid was collected by filtration and the target product Co(L3) (11.6 g, red solid) was obtained after drying in 96% yield. | [References]
[1] Patent: US2006/173210, 2006, A1. Location in patent: Page/Page column 5 |
| Questions And Answer | Back Directory | [Reaction]
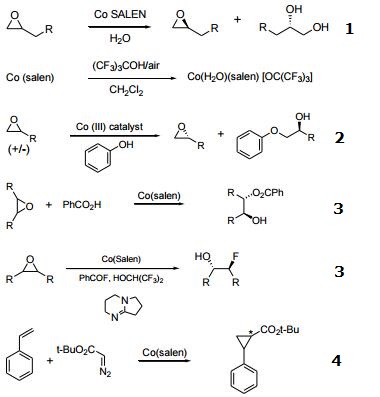
- Catalyst used in the kinetic resolution of racemic, terminal epoxides yielding a chiral diol and the unreacted enantiomer of the epoxide.
- Precursor to a Co(III) catalyst for the kinetic resolution of terminal epoxides with alcohols.
- Desymmetrization of meso-epoxides with carboxylic acids and fluoride.
- Catalyst for asymmetric cyclopropanation of styrene.
- Catalyst for copolymerization of CO2 and epoxides.
- Enantioselective intramolecular openings of oxetanes.

|
|
|