| Identification | More | [Name]
Cyanuric fluoride | [CAS]
675-14-9 | [Synonyms]
2,4,6-TRIFLUORO-1,3,5-TRIAZINE
CYANURIC FLUORIDE
1,3,5-triazine,2,4,6-trifluoro-
2,4,6-trifluoro-s-triazin
2,4,6-Trifluoro-s-triazine
2,4,6-Trifluorotriazine
2,4,6-trifluro-s-triazin
5-triazine,2,4,6-trifluoro-3
Cyanuric trifluoride
s-Triazine, 2,4,6-trifluoro-
Trifluoro-1,3,5-triazine
Trifluoro-s-triazine
Trifluorotriazine
Cyanuricfluoride,98%
Cyanuric fluoride 98%
Cyanuric fluoride: (2,4,6-Trifluoro-s-triazine)
1,3,5-Trifluoro-2,4,6-triazine | [EINECS(EC#)]
211-620-8 | [Molecular Formula]
C3F3N3 | [MDL Number]
MFCD00014597 | [Molecular Weight]
135.05 | [MOL File]
675-14-9.mol |
| Chemical Properties | Back Directory | [Melting point ]
-38°C | [Boiling point ]
73-74°C | [density ]
1,6 g/cm3 | [refractive index ]
1.348 | [Fp ]
73-74°C | [storage temp. ]
Hygroscopic, Refrigerator, under inert atmosphere | [solubility ]
Miscible with chloroform, carbon tetrachloride,ether, dioxane and ketones. | [form ]
Oil | [pka]
-2.62±0.10(Predicted) | [color ]
Clear Colourless | [Sensitive ]
Moisture Sensitive | [BRN ]
124237 | [Exposure limits]
ACGIH: TWA 2.5 mg/m3
NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 | [CAS DataBase Reference]
675-14-9(CAS DataBase Reference) | [NIST Chemistry Reference]
1,3,5-Triazine, 2,4,6-trifluoro-(675-14-9) | [EPA Substance Registry System]
675-14-9(EPA Substance) |
| Questions And Answer | Back Directory | [Synthesis]
HF offers the attraction of cheapness, although this may be offset by its handling
difficulties and lack of reactivity due to H-bonding. However, it may be used to
fluorinate very reactive substrates. Trichlorotriazine may be converted to
trifluorotriazine.
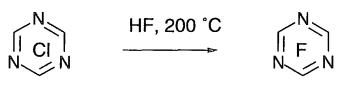 |
| Safety Data | Back Directory | [Hazard Codes ]
T+,C,T | [Risk Statements ]
R24:Toxic in contact with skin.
R26:Very Toxic by inhalation.
R35:Causes severe burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3389 6.1/PG 1
| [WGK Germany ]
3
| [RTECS ]
XZ1750000
| [F ]
10 | [Hazard Note ]
Corrosive/Toxic | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
I | [Hazardous Substances Data]
675-14-9(Hazardous Substances Data) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Cyanuric chloride-->Polyethylene-->HYDROGEN FLUORIDE GAS | [Preparation Products]
2-[[6-[[6-anilino-4-fluoro-1,3,5-triazin-2-yl]amino]-1-hydroxy-3-sulpho-2-naphthyl]azo]-5-methoxybenzene-1,4-disulphonic acid, sodium salt-->Reactive Blue 94-->2,7-Naphthalenedisulfonic acid, 5-(benzoylamino)-3-5-4-fluoro-6-(1-sulfo-2-naphthalenyl)amino-1,3,5-triazin-2-ylamino-2-sulfophenylazo-4-hydroxy-, tetrasodium salt-->Reactive orange 91-->N,N,N',N'-Tetraethyl-6-fluoro-1,3,5-triazine-2,4-diamine |
| Hazard Information | Back Directory | [General Description]
Liquid. | [Reactivity Profile]
Inorganic oxidizing agents can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates. Explosives often consist of an inorganic oxidizing agent mixed in intimate contact with a reducing agent. Gunpowder is such a mixture. Other examples are a mixture of sugar (an organic compound) plus sodium chlorate and magnesium (an inorganic reducing agent) plus barium peroxide. Compounds that inherently contain a group that is a reducing agent and an oxidizing agent are classed in both Group 44 (Inorganic Oxidizing Agents) and in Group 45 (Inorganic Reducing Agents; for example, ammonium nitrate). The strongly oxidizing elements oxygen and fluorine are classified here. Inorganic oxidizing agents that are also acids (such as nitric and perchloric acids) are not included in this group. They are in Group 2 (Acids, Inorganic Oxidizing). | [Health Hazard]
This material is highly toxic by skin contact and inhalation. | [Fire Hazard]
When heated to decomposition, CYANURIC FLUORIDE emits very toxic fumes of fluorides and nitrogen oxides. Avoid decomposing heat. | [Chemical Properties]
Colorless liquid. Decompose violently with water and alcohol. | [Characteristics]
Cyanuric fluoride is a key intermediate in the synthesis of fluorine homotriazine reactive dyes. Compared with the traditional chlorotriazine reactive dyestuff, fluorine homotriazine reactive dyestuff has the following five advantages:
1. Energy saving, low dyeing temperature (medium temperature type) 60℃, lower than KE type reactive dyestuff and comparable to M type reactive dyestuff.
2. Emission reduction, environmental protection, high fixation rate, 15-20 percentage points higher than the corresponding chlorotriazine reactive dyestuff fixation rate, can improve the utilization rate of dyestuff, but also reduce the environmental pollution in the printing and dyeing process.
3. Good stability of dyestuff.
4. High stability of peroxide resistance.
5. Not restricted by AOX regulations. | [Uses]
Cyanuric fluoride acts as a fluorinating agent used in the conversion of carboxylic acids into acyl fluorides. It is used as a precursor for fibre-reactive dyes. It is a specific reagent for tyrosine residues in enzyme. Further, it is involved in the preparation of cyanuric acid by hydrolysis | [Preparation]
In a dry three-necked flask equipped with stirrer, thermometer and reflux condenser, add 0.2mol (36.9g) of cyanuric chloride, 0.63mol (36.5g) of finely ground anhydrous potassium fluoride, 60mL of xylene and 2.0g of polyethylene glycol-600, heat and stir at 110℃, reflux for 16h and distill after a little cooling, collect the fraction at 72℃~75℃, and get liquid of cyanuric trioxide. |
|
|