1032823-75-8
 1032823-75-8 结构式
1032823-75-8 结构式
基本信息
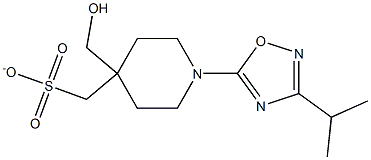
5-[[[1-(3-异丙基-1,2,4-二唑-5-基)-4-哌啶基]甲基]氧基]-2-[4-(甲磺酰基)苯基]吡啶
3-异丙基-5-(4-(((6-(4-(甲基磺酰)苯基)吡啶-3-基)氧基)甲基)哌啶-1-基)-1,2,4-恶二唑
5-[[[1-(3-异丙基-1,2,4-噁二唑-5-基)-4-哌啶基]甲基]氧基]-2-[4-(甲磺酰基)苯基]吡啶
5-[[[1-(3-异丙基-1,2,4-恶二唑-5-基)-4-哌啶基]甲基]氧基]-2-[4-(甲磺酰基)苯基]吡啶
GSK1292263
GSK1292263 USP/EP/BP
GSK-1292263
GSK 1292263
GPR119 receptor agonist GS1292263
3-Isopropyl-5-(4-(((6-(4-(methylsulfonyl)phenyl)pyridin-3-yl)oxy)methyl)piperidin-1-yl)-1,2,4-
3-Isopropyl-5-(4-(((6-(4-(methylsulfonyl)phenyl)pyridin-3-yl)oxy)methyl)piperidin-1-yl)-1,2,4-oxa
5-((1-(3-isopropyl-1,2,4-oxadiazol-5-yl)piperidin-4-yl)methoxy)-2-(4-(methylsulfonyl)phenyl)pyridine
3-isopropyl-5-(4-(((6-(4-(Methylsulfonyl)phenyl)pyridin-3-yl)oxy)Methyl)piperidin-1-yl)-1,2,4-oxadiazole
Pyridine, 5-[[1-[3-(1-methylethyl)-1,2,4-oxadiazol-5-yl]-4-piperidinyl]methoxy]-2-[4-(methylsulfonyl)phenyl]-
物理化学性质
制备方法
1032825-19-6

1032825-20-9

1032823-75-8
以1-[3-(1-甲基乙基)-1,2,4-恶二唑-5-基]-4-哌啶基甲基甲磺酸酯(82.3g,271mmol)和5-羟基-2-(4-甲基磺酰基苯基)吡啶(71.0g,285mmol)为原料,加入粉末状碳酸钾(118g,855mmol)和N,N-二甲基甲酰胺(750mL),在氮气保护下于80℃机械搅拌反应20小时。反应完成后,冷却至室温,倒入冰水(3L)中,静置1小时。过滤收集固体产物,用水(2×500mL)洗涤,空气干燥。将干燥后的固体溶于二氯甲烷(300mL)和甲醇(500mL)的混合溶剂中。通过旋转蒸发仪在55℃下缓慢蒸除二氯甲烷。将剩余的甲醇溶液在室温下静置16小时,促使结晶。过滤得到结晶固体,用冷甲醇洗涤,60℃真空干燥18小时,得到目标化合物3-异丙基-5-(4-(((6-(4-(甲基磺酰)苯基)吡啶-3-基)氧基)甲基)哌啶-1-基)-1,2,4-恶二唑(105.7g,收率84%),为浅棕褐色固体。1H NMR(400MHz,CDCl3):δ8.41(d,1H,J=2.8Hz),8.13(d,2H,J=8.6Hz),8.01(d,2H,J=8.6Hz),7.74(d,1H,J=8.7Hz),7.29(dd,1H,Ja=8.7Hz,Jb=3.0Hz),4.24(d,2H,J=13.1Hz),3.95(d,2H,J=6.2Hz),3.17-3.04(m,5H),2.94-2.84(m,1H),2.11(bs,1H),1.97(d,2H,J=12.6Hz),1.54-1.42(m,2H),1.29(d,6H,J=7.0Hz);LRMS(ESI),m/z 457(M+H)。
参考文献:
[1] Patent: US2010/29650, 2010, A1. Location in patent: Page/Page column 50
常见问题列表
| Target | Value |
|
GPR119
() |
GSK-1292263通过使用Hypo1从1538种化合物中被选出,与Hypo1中一致的GSK-1292263的拟合值和估计值分别为8.8和7.7 (nM)。
